Cohorts of premedication for endoscopy of the upper gastrointestinal tract with simethicone, N-acetylcysteine, Hedera helix and visual scale validation
DOI:
https://doi.org/10.22516/25007440.582Keywords:
Premedication, Simethicone, N-acetylcysteine, Esophagogastroduodenoscopy, TVMS, Interobserver agreementAbstract
Quality parameters for upper gastrointestinal endoscopy have introduced intraprocedural indicators, including adequate mucosal visualization free of saliva, mucus, or bubbles, which may increase the possibility of early-stage injury detection. The use of mucolytics and anti-foaming agents has shown great efficiency variability depending on the type of solution, concentrations, exposure times and visibility scale applied.
Objectives: To determine the effectiveness of different premedication solutions for cleaning the digestive mucosa; to validate, by means of an interobserver concordance test, a new scale for the adequate visualization of the mucosa (TVMS) for the esophagus, stomach, and duodenum; and to report adverse events or complications associated with the solutions used and the procedures performed.
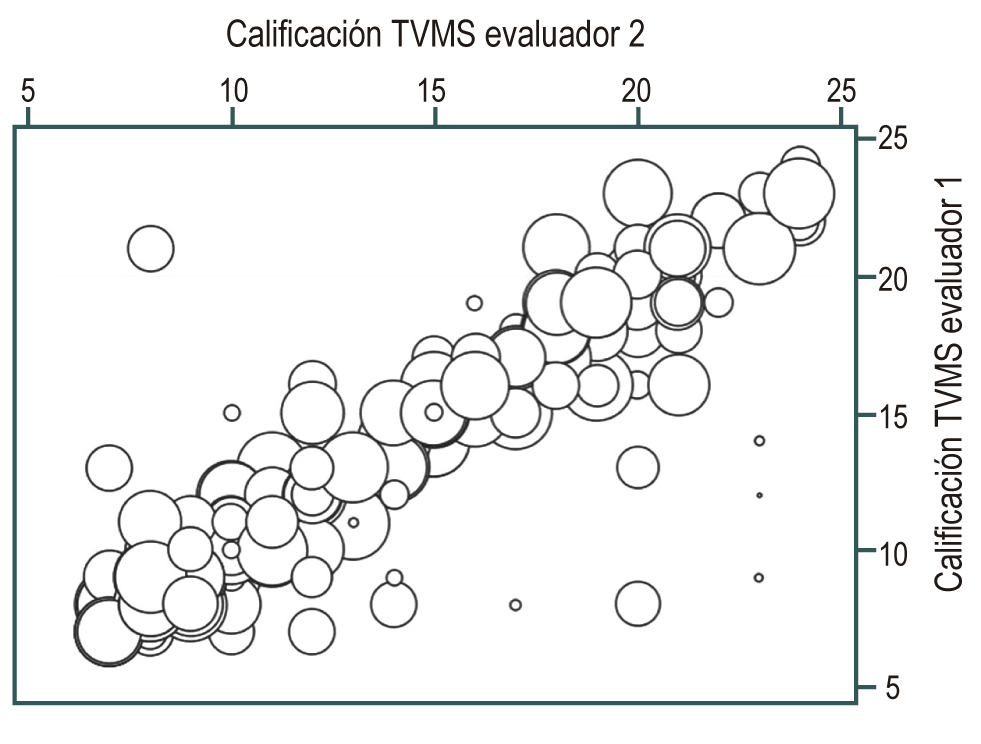
Material and methods: Prospective, comparative cohort study. 412 adult patients, ASA I and ASA II, were included for diagnostic endoscopy under conscious sedation. They were distributed in 6 similar cohorts and divided into two groups: non-premedication, 2 in C1 (fasting 6 to 8 hours) and C2 (water 100 mL) cohorts; premedication, 4 C3 to C6 cohorts (C3: water 100 mL + simethicone 1000 mg; C4: water 100 ml + simethicone 200 mg + N-acetylcysteine 600 mg; C5: water 100 ml + simethicone 200 mg + N-acetylcysteine 1000 mg; C6: water 100 ml + simethicone 200 mg + Hedera helix 70 mg). The solution was swallowed 15 to 30 minutes passing through the cricopharyngeus muscle. The Kappa test was performed to measure interobserver concordance of the TVMS scale.
Results: Of 412 patients, 58 % were female; 23 % (136) were included in the C1 and C2 cohorts; and 67 % (276) were in the C3 to C6 cohorts. The average exposure time to each solution was 24.4 minutes. The wash volume for proper visualization was significantly different between the two groups. In premedicated patients, 75.6 mL of solution were used, while in patients without premedication, 124 mL were used (p = 0.000), with an excellent quality of TVMS of 88.7% versus 41.4%, respectively. The C4 cohort (water 100 mL + simethicone 200 mg + N-acetylcysteine 600 mg) was the most effective with a significant difference (p = 0.001) compared with the C1 (fasting) and C2 (placebo with water 100 mL) cohorts. It also had better efficiency compared to the C3, C5 and C6 cohorts in that order. There were no adverse events or complications associated with endoscopy, sedation, or premedication products.
Conclusions: The most effective solution as a premedication to achieve excellent visibility of the digestive mucosa was that used in the C4 cohort (SIM 200 + NAC 600 + H2OR 100 mL). The proposed TVMS scale is a very complete and easy tool to apply by more than one observer. Premedication ingested, with anti-foam, mucolytic and water up to 100 mL, between 15 and 30 minutes before endoscopy, is safe under the conditions described in this study.
Downloads
References
Adams MA, Saini SD, Allen JI. Quality measures in gastrointestinal endoscopy: the current state. Curr Opin Gastroenterol. 2017;33(5):352-357. doi: 10.1097/MOG.0000000000000379
Bisschops R, Areia M, Coron E, Dobru D, Kaskas B, Kuvaev R, Pech O, Ragunath K, Weusten B, Familiari P, Domagk D, Valori R, Kaminski MF, Spada C, Bretthauer M, Bennett C, Senore C, Dinis-Ribeiro M, Rutter MD. Performance measures for upper gastrointestinal endoscopy: A European Society of Gastrointestinal Endoscopy quality improvement initiative. United European Gastroenterol J. 2016;4(5):629-656. doi: 10.1177/2050640616664843
Beg S, Ragunath K, Wyman A, Banks M, Trudgill N, Pritchard DM, Riley S, Anderson J, Griffiths H, Bhandari P, Kaye P, Veitch A. Quality standards in upper gastrointestinal endoscopy: a position statement of the British Society of Gastroenterology (BSG) and Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUGIS). Gut. 2017;66(11):1886-1899. doi: 10.1136/gutjnl-2017-314109
Koga M, Arakawa K. [On the application of enzymatic mucinolysis in x-ray diagnosis of the stomach]. Nihon Igaku Hoshasen Gakkai Zasshi. 1964;24:1011-31.
Ida K, Okuda J, Nakazawa S, Yoshino J, Itoh M, Yokoyama Y, et al. Clinical evaluation of premedication with KPD (Pronase) in gastroendoscopy-placebo-controlled double blind study in dye scatt ering endoscopy. Clin Rep. 1991;25:1793-804.
Monrroy H, Vargas JI, Glasinovic E, Candia R, Azúa E, Gálvez C, Rojas C, Cabrera N, Vidaurre J, Álvarez N, González J, Espino A, González R, Parra-Blanco A. Use of N-acetylcysteine plus simethicone to improve mucosal visibility during upper GI endoscopy: a double-blind, randomized controlled trial. Gastrointest Endosc. 2018;87(4):986-993. doi: 10.1016/j.gie.2017.10.005
Elvas L, Areia M, Brito D, Alves S, Saraiva S, Cadime AT. Premedication with simethicone and N-acetylcysteine in improving visibility during upper endoscopy: a double-blind randomized trial. Endoscopy. 2017;49(2):139-145. doi: 10.1055/s-0042-119034
Callaghan JL, Neale JR, Boger PC, Sampson AP, Patel P. Variation in preparation for gastroscopy: lessons towards safer and better outcomes. Frontline Gastroenterol. 2016;7(3):187-190. doi: 10.1136/flgastro-2015-100647
Song M, Kwek AB, Law NM, Ong JP, Tan JY, Harichander Thurairajah P, Ang DS, Ang TL. Efficacy of small-volume simethicone given at least 30 min before gastroscopy. World J Gastrointest Pharmacol Ther. 2016;7(4):572-578. doi: 10.4292/wjgpt.v7.i4.572
Bertoni G, Gumina C, Conigliaro R, Ricci E, Staffetti J, Mortilla MG, Pacchione D. Randomized placebo-controlled trial of oral liquid simethicone prior to upper gastrointestinal endoscopy. Endoscopy. 1992;24(4):268-70. doi: 10.1055/s-2007-1010479
Sajid MS, Rehman S, Chedgy F, Singh KK. Improving the mucosal visualization at gastroscopy: a systematic review and meta-analysis of randomized, controlled trials reporting the role of Simethicone ± N-acetylcysteine. Transl Gastroenterol Hepatol. 2018;3:29. doi: 10.21037/tgh.2018.05.02
Li Y, Du F, Fu D. The effect of using simethicone with or without N-acetylcysteine before gastroscopy: A meta-analysis and systemic review. Saudi J Gastroenterol. 2019 Jul-;25(4):218-228. doi: 10.4103/sjg.SJG_538_18
Zhang LY, Li WY, Ji M, Liu FK, Chen GY, Wu SS, Hao Q, Zhai HH, Zhang ST. Efficacy and safety of using premedication with simethicone/Pronase during upper gastrointestinal endoscopy examination with sedation: A single center, prospective, single blinded, randomized controlled trial. Dig Endosc. 2018;30(1):57-64. doi: 10.1111/den.12952
Mansilla R, Uslar T, Chahuán J, Latorre G, Cruz R, Cruz R, Sirhan M, Espino A, Honold F, Huenur J, Miranda P, Riquelme A. Validez y confiabilidad de una escala de clasificación de limpieza gástrica en endoscopia digestiva. Gastroenterol Latinoam. 2016;27(1): 9-17.
Chang WK, Yeh MK, Hsu HC, Chen HW, Hu MK. Efficacy of simethicone and N-acetylcysteine as premedication in improving visibility during upper endoscopy. J Gastroenterol Hepatol. 2014;29(4):769-74. doi: 10.1111/jgh.12487
Buxbaum JL, Hormozdi D, Dinis-Ribeiro M, Lane C, Dias-Silva D, Sahakian A, Jayaram P, Pimentel-Nunes P, Shue D, Pepper M, Cho D, Laine L. Narrow-band imaging versus white light versus mapping biopsy for gastric intestinal metaplasia: a prospective blinded trial. Gastrointest Endosc. 2017 Nov;86(5):857-865. doi: 10.1016/j.gie.2017.03.1528
World Source: Globocan 2020 [Internet]. IARC; 2020 [citado el 5 de febrero de 2020]. Disponible en: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf
Wiesner Ceballos C, Henríquez Mendoza GM, Aguilera López J. Análisis de situación del cáncer en Colombia: 2015. Colombia, Ministerio de Salud, Instituto Nacional de Cancerología; 2017. Disponible en: https://www.cancer.gov.co/Situacion_del_Cancer_en_Colombia_2015.pdf
Matsuzaka M, Fukuda S, Takahashi I, Shimaya S, Oyama T, Yaegaki M, Shimoyama T, Sakamoto J, Nakaji S, Umeda T. The decreasing burden of gastric cancer in Japan. Tohoku J Exp Med. 2007;212(3):207-19. doi: 10.1620/tjem.212.207
Kim GH, Liang PS, Bang SJ, Hwang JH. Screening and surveillance for gastric cancer in the United States: Is it needed? Gastrointest Endosc. 2016;84(1):18-28. doi: 10.1016/j.gie.2016.02.028
Gómez-Zuleta MA, Ruíz-Morales OF, Otero-Regino W. Efectividad de la premedicación con N-acetil cisteína más dimetilpolisiloxano versus un placebo para mejorar la visibilidad en la endoscopia digestiva: estudio prospectivo, ciego, controlado aleatorizado. Medicina (Mex). 2017;39(2):98-106.
Royero Gutiérrez HA. Aplicación de una escala de visualización de la mucosa gástrica, durante la esofagogastroduodenoscopia en pacientes premedicados con N-acetilcisteína más simeticona: experiencia en Ocaña, Norte de Santander. Rev Colomb Gastroenterol. 2018;33(1):1-7. https://doi.org/10.22516/25007440.226
Blanco Avellaneda CJ, García KR, Molano A, Chimbi DY, Forero AY. Seguridad y eficiencia de sedación balanceada con propofol y remifentanil en endoscopia digestiva alta diagnóstica. Una experiencia exitosa. Rev Colomb Gastroenterol. 2017;32(2):120-30. https://doi.org/10.22516/25007440.140
McNally PR, Maydonovitch CL, Wong RK. The effectiveness of simethicone in improving visibility during colonoscopy: a double-blind randomized study. Gastrointest Endosc. 1988;34(3):255-8. doi: 10.1016/s0016-5107(88)71324-3
Bhandari P, Green S, Hamanaka H, Nakajima T, Matsuda T, Saito Y, Oda I, Gotoda T. Use of Gascon and Pronase either as a pre-endoscopic drink or as targeted endoscopic flushes to improve visibility during gastroscopy: a prospective, randomized, controlled, blinded trial. Scand J Gastroenterol. 2010;45(3):357-61. doi: 10.3109/00365520903483643
Lee GJ, Park SJ, Kim SJ, Kim HH, Park MI, Moon W. Effectiveness of Premedication with Pronase for Visualization of the Mucosa during Endoscopy: A Randomized, Controlled Trial. Clin Endosc. 2012;45(2):161-4. doi: 10.5946/ce.2012.45.2.161
Bertoni G, Gumina C, Conigliaro R, Ricci E, Staffetti J, Mortilla MG, Pacchione D. Randomized placebo-controlled trial of oral liquid simethicone prior to upper gastrointestinal endoscopy. Endoscopy. 1992;24(4):268-70. doi: 10.1055/s-2007-1010479
Kuo CH, Sheu BS, Kao AW, Wu CH, Chuang CH. A defoaming agent should be used with pronase premedication to improve visibility in upper gastrointestinal endoscopy. Endoscopy. 2002;34(7):531-4. doi: 10.1055/s-2002-33220
Asl SM, Sivandzadeh GR. Efficacy of premedication with activated Dimethicone or N-acetylcysteine in improving visibility during upper endoscopy. World J Gastroenterol. 2011;17(37):4213-7. doi: 10.3748/wjg.v17.i37.4213
Chang CC, Chen SH, Lin CP, Hsieh CR, Lou HY, Suk FM, Pan S, Wu MS, Chen JN, Chen YF. Premedication with pronase or N-acetylcysteine improves visibility during gastroendoscopy: an endoscopist-blinded, prospective, randomized study. World J Gastroenterol. 2007;13(3):444-7. doi: 10.3748/wjg.v13.i3.444
Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, Esposito G, Garrido M, Kikuste I, Megraud F, Matysiak-Budnik T, Annibale B, Dumonceau JM, Barros R, Fléjou JF, Carneiro F, van Hooft JE, Kuipers EJ, Dinis-Ribeiro M. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51(4):365-388. doi: 10.1055/a-0859-1883
Koeppe AT, Lubini M, Bonadeo NM, Moraes I Jr, Fornari F. Comfort, safety and quality of upper gastrointestinal endoscopy after 2 hours fasting: a randomized controlled trial. BMC Gastroenterol. 2013;13:158. doi: 10.1186/1471-230X-13-158
De Silva AP, Amarasiri L, Liyanage MN, Kottachchi D, Dassanayake AS, de Silva HJ. One-hour fast for water and six-hour fast for solids prior to endoscopy provides good endoscopic vision and results in minimum patient discomfort. J Gastroenterol Hepatol. 2009;24(6):1095-7. doi: 10.1111/j.1440-1746.2009.05782.x
Ofstead CL, Wetzler HP, Johnson EA, Heymann OL, Maust TJ, Shaw MJ. Simethicone residue remains inside gastrointestinal endoscopes despite reprocessing. Am J Infect Control. 2016 Nov 1;44(11):1237-1240. doi: 10.1016/j.ajic.2016.05.016
Sutton S, Jimenez L. A Review of Reported Recalls Involving Microbiological Control 2004-2011 with Emphasis on FDA Considerations of “Objectionable Organisms” [Internet]. American Pharmaceutical Review; 2012 [citado el 21 de febrero de 2020]. Disponible en: http://www.americanpharmaceuticalreview.com/Featured-Articles/38382-A-Review-of-Reported-Recalls-Involving-Microbiological-Control-2004-2011-with-Emphasis-on-FDA-Considerations-of-Objectionable-Organisms/
Devereaux BM, Taylor ACF, Athan E, Wallis DJ, Brown RR, Greig SM, Bailey FK, Vickery K, Wardle E, Jones DM. Simethicone use during gastrointestinal endoscopy: Position statement of the Gastroenterological Society of Australia. J Gastroenterol Hepatol. 2019;34(12):2086-2089. doi: 10.1111/jgh.14757
Downloads
Published
How to Cite
Issue
Section
License
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
Los autores/as ceden sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los contenidos están protegidos bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.

| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |















