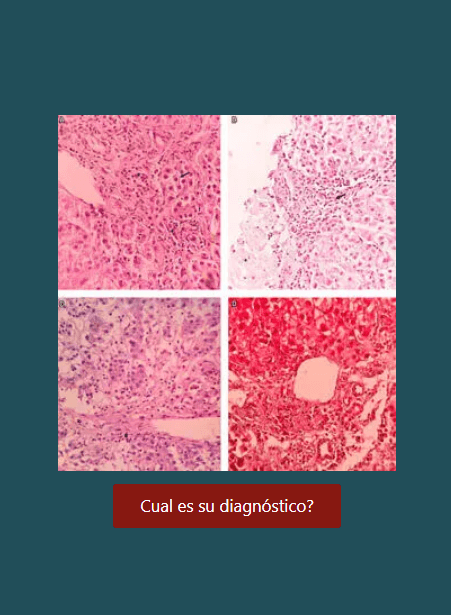
Enfermedades hepáticas y embarazo
DOI:
https://doi.org/10.22516/25007440.367Palavras-chave:
embarazo, hiperémesis gravídica, colestasis gestacional, HELL, preeclampsia, virus de la hepatitis B, cirrosis, trasplante hepáticoResumo
La prevalencia de las enfermedades hepáticas en el embarazo no es despreciable, ya que estas se presentan en 3%-5% de todas las gestaciones. Entre las múltiples causas se encuentran cambios fisiológicos del embarazo; enfermedad hepática preexistente, siendo las más comunes las enfermedades colestásicas (colangitis biliar primaria y colangitis esclerosante primaria), hepatitis autoinmune, enfermedad de Wilson, hepatitis virales crónicas, cirrosis establecida de cualquier etiología y paciente con historia de trasplante hepático; enfermedad hepática adquirida durante el embarazo, siendo las principales las hepatitis virales, la toxicidad inducida por medicamentos y la hepatolitiasis; hepatopatía relacionada con el embarazo, en la cual se encuentran 5 entidades principales: hiperémesis gravídica, colestasis intrahepática del embarazo, preeclampsia, síndrome HELLP e hígado graso del embarazo.
La severidad de estas entidades tiene una amplia gama de presentaciones, desde la paciente que es completamente asintomática, hasta la falla hepática aguda e incluso la muerte. La gravedad del cuadro se asocia con una morbilidad y mortalidad significativas tanto para la madre como para el feto, lo cual hace que una evaluación rápida, diagnóstico certero y manejo apropiado por un equipo multidisciplinario (incluida obstetricia de alto riesgo, hepatología, gastroenterología y radiología intervencionista), en un servicio que tenga la posibilidad de ofrecer trasplante hepático, sean fundamentales para obtener buenos desenlaces.
Downloads
Referências
Lai M, Wolf J. The liver in pregnancy. Handbook of Liver Disease. Elsevier. 2018. p. 308-23. Disponible en: https://doi.org/10.1016/B978-0-323-47874-8.00023-7.
Westbrook R, Dusheiko G, Williamson C. Pregnancy and liver disease. J Hepatol. 2016;64(4):933-45. doi: https://doi.org/10.1016/j.jhep.2015.11.030.
Shekhar S, Diddi G. Liver disease in pregnancy. Taiwan J Obstet Gynecol. 2015;54(5):475-82. doi: https://doi.org/10.1016/j.tjog.2015.01.004.
Than N, Neuberger J. Liver abnormalities in pregnancy. Best Pract Res Clin Gastroenterol. 2013;27(4):565-75. doi: https://doi.org/10.1016/j.bpg.2013.06.015.
Clinical management guidelines for obstetrician-gynecologists. Number 153, September 2015: (replaces practice bulletin number 52, April 2004). Obstet Gynecol. 2003;126(12):12-24.
London V, Grube S, Sherer D, Abulafia O. Hyperemesis gravidarum: a review of recent literature. Pharmacology. 2017;100(3-4):161-71. doi: https://doi.org/10.1159/000477853.
Westbrook R, Yeoman A, O'Grady J, Harrison P, Devlin J, Heneghan M. Model for end-stage liver disease score predicts outcome in cirrhotic patients during pregnancy. Clin Gastroenterol Hepatol. 2011;9(8):694-9. doi: https://doi.org/10.1016/j.cgh.2011.03.036.
Deepak J, Andra J, Quaglia A, Westbrook R, Heneghan M. Liver disease in pregnancy. Lancet. 2010;375:594-605. doi: https://doi.org/10.1016/S0140-6736(09)61495-1.
Management of hyperemesis gravidarum. Drug Ther Bull. 2013;51(11):126-9. doi: https://doi.org/10.1136/dtb.2013.11.0215.
Aggarwal N, Negi N, Aggarwal A, Bodh V, Dhiman R. Pregnancy with portal hypertension. J Clin Exp Hepatol. 2014;4(2):163-71. doi: https://doi.org/10.1016/j.jceh.2014.05.014.
Tran T, Ahn J, Reau N. ACG clinical guideline: liver disease and pregnancy. Am J Gastroenterol. 2016;111(2):176-94. doi: https://doi.org/10.1038/ajg.2015.430.
Geenes V, Williamson C. Gastrointestinal and liver disease in pregnancy. Obstet Gynaecol Reprod Med. 2017;27(3):91-8. doi: https://doi.org/10.1016/j.ogrm.2017.01.005.
Frise C, Williamson C. Liver disease in pregnancy. Medicine (Baltimore). 2015;43(11):636-8. doi: https://doi.org/10.1016/j.mpmed.2015.08.010.
Geenes V, Williamson C. Liver disease in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2015;29(5):612-24. doi: https://doi.org/10.1016/j.bpobgyn.2015.04.003.
Ditisheim A, Sibai B. Diagnosis and management of HELLP syndrome complicated by liver hematoma. Clin Obstet Gynecol. 2017;60(1):190-7. doi: https://doi.org/10.1097/GRF.0000000000000253.
Liu J, Ghaziani T, Wolf L. Acute fatty liver disease of pregnancy: updates in pathogenesis, diagnosis, and management. Am J Gastroenterol. 2017;112(6):838-46. doi: https://doi.org/10.1038/ajg.2017.54.
Knight M, Nelson-Piercy C, Kurinczuk J, Spark P, Brocklehurst P, UK Obstetric Surveillance System. A prospective national study of acute fatty liver of pregnancy in the UK. Gut. 2008;57(7):951-6. doi: https://doi.org/10.1136/gut.2008.148676.
Papafragkakis H, Singhal S, Anand S. Acute fatty liver of pregnancy. South Med J. 2013;106(10):588-93. doi: https://doi.org/10.1097/SMJ.0000000000000007.
Buytaert I, Elewaut G, van Kets H. Early occurrence of acute fatty liver in pregnancy. Am J Gastroenterol. 1996;91(3):603-4.
Natarajan S, Ibdah J. Role of 3-hydroxy fatty acid-induced hepatic lipotoxicity in acute fatty liver of pregnancy. Int J Mol Sci. 2018;19(1):1-17. doi: https://doi.org/10.3390/ijms19010322.
Lee N, Brady C. Liver disease in pregnancy. World J Gastroenterol. 2009;15(8):897-906. doi: https://doi.org/10.3748/wjg.15.897.
Steingrub J. Pregnancy-associated severe liver dysfunction. Crit Care Clin. 2004;20(4):763-76. doi: https://doi.org/10.1016/j.ccc.2004.05.006.
Ch'ng C, Morgan M, Hainsworth I, Kingham J. Prospective study of liver dysfunction in pregnancy in Southwest Wales. Gut. 2002;51(6):876-80. doi: https://doi.org/10.1136/gut.51.6.876.
Maléth J, Venglovecz V, Rázga Z, Tiszlavicz L, Rakonczay Z, Hegyi P. Non-conjugated chenodeoxycholate induces severe mitochondrial damage and inhibits bicarbonate transport in pancreatic duct cells. Gut. 2011;60(1):136-8. doi: https://doi.org/10.1136/gut.2009.192153.
Remiszewski P, Pawlak J, Skwarek A, Grzelak I, Patkowski W, Grodzicki M, et al. Orthotopic liver transplantation for acute liver failure resulting from «acute fatty liver of pregnancy». Ann Transplant. 2003;8(3):8-11.
Wei Q, Zhang L, Liu X. Clinical diagnosis and treatment of acute fatty liver of pregnancy: a literature review and 11 new cases. J Obstet Gynaecol Res. 2010;36(4):751-6. doi: https://doi.org/10.1111/j.1447-0756.2010.01242.x.
Westbrook R, Yeoman A, Joshi D, Heaton N, Quaglia A, OGrady JG, et al. Outcomes of severe pregnancy-related liver disease: refining the role of transplantation. Am J Transplant. 2010;10(11):2520-6. doi: https://doi.org/10.1111/j.1600-6143.2010.03301.x.
Gatselis N, Zachou K, Koukoulis G, Dalekos G. Autoimmune hepatitis, one disease with many faces: etiopathogenetic, clinico-laboratory and histological characteristics. World J Gastroenterol. 2015;21(1):60. doi: https://doi.org/10.3748/wjg.v21.i1.60.
Hennes E, Zeniya M, Czaja A, Parés A, Dalekos G, Krawitt EL, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48(1):169-76. doi: https://doi.org/10.1002/hep.22322.
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: autoimmune hepatitis. J Hepatol. 2015;63(4):971-1004. doi: https://doi.org/10.1016/j.jhep.2015.06.030.
Manns M, Czaja A, Gorham J, Krawitt E, Mieli-Vergani G, Vergani D, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51(6):2193-213. doi: https://doi.org/10.1002/hep.23584.
Almashhrawi A, Khulood A, Rubayat R, Ghassan H, Jamal I. Liver diseases in pregnancy: diseases not unique to pregnancy. World J Gastroenterol. 2013;19(43):7630. doi: https://doi.org/10.3748/wjg.v19.i43.7630.
Braga A, Vasconcelos C, Braga J. Pregnancy with autoimmune hepatitis. Bed Bench. 2016;9(3):220-4.
Schmeltzer P, Russo M. Clinical narrative: autoimmune hepatitis. Am J Gastroenterol. 2018;113(7):951-8. doi: https://doi.org/10.1038/s41395-018-0058-z.
Schramm C, Herkel J, Beuers U, Kanzler S, Galle P, Lohse A. Pregnancy in autoimmune hepatitis: outcome and risk factors. Am J Gastroenterol. 2006;101(3):556-60. doi: 10.1111/j.1572-0241.2006.00479.x.
Orgul G, Ozkan E, Celik H, Beksac M. Autoimmune hepatitis and pregnancy: report of two cases with different maternal outcomes. Clin Exp Hepatol. 2017;4:212-4. doi: https://doi.org/10.5114/ceh.2017.71445.
Heneghan M, Norris S, O´Grady J, Harrison P, McFalane I. Management and outcome of pregnancy in autoimmune hepatitis. Gut. 2001;48(1):97-102. doi: https://doi.org/10.1136/gut.48.1.97.
Westbrook R, Yeoman A, Kriese S, Heneghan M. Outcomes of pregnancy in women with autoimmune hepatitis. J Autoimmun. 2012;38(2-3):J239-44. doi: https://doi.org/10.1016/j.jaut.2011.12.002.
Bremer L, Schramm C, Tiegs G. Immunology of hepatic diseases during pregnancy. Semin Immunopathol. 2016;38(6):669-85. doi: https://doi.org/10.1007/s00281-016-0573-1.
Sebode M, Schramm C. AIH: which alternative for difficult-to-treat patients? Dig Dis. 2015;33(2):83-7. doi: https://doi.org/10.1159/000440752.
Lammert C, Loy V, Oshima K, Gawrieh S. Management of difficult cases of autoimmune hepatitis. Curr Gastroenterol Rep. 2016;18(2). doi: http://link.springer.com/10.1007/s11894-015-0484-7.
Terrault N, Bzowej N, Chang KM, Hwang J, Jonas M, Murad M, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatol Baltim Md. 2016;63(1):261-83. doi: https://doi.org/10.1002/hep.28156.
Association E. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57(1):167-85. doi: https://doi.org/10.1016/j.jhep.2012.02.010.
Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. doi: https://doi.org/10.1046/j.1365-2893.2003.00487.x.
Tran T. Hepatitis B in pregnancy. Clin Infect Dis. 2016;62(4):S314-7. doi: https://doi.org/10.1093/cid/ciw092.
Dionne-Odom J, Tita A, Silverman N. Hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214(6-14).
Patton H, Tran T. Management of hepatitis B during pregnancy. Nat Rev Gastroenterol Hepatol. 2014;11(7):402-9. doi: https://doi.org/10.1038/nrgastro.2014.30.
Rac M, Sheffield J. Prevention and management of viral hepatitis in pregnancy. Obstet Gynecol Clin N Am. 2014;41:573-92. doi: https://doi.org/10.1016/j.ogc.2014.08.004.
Castillo E, Murphy K, van Schalkwyk J. Hepatitis B and pregnancy. J Obstet Gynaecol Can JOGC J. 2017;39(3):181-90. doi: https://doi.org/10.1016/j.jogc.2016.11.001.
Liu CP, Zeng YL, Zhou M, Chen LL, Hu R, Wang L, et al. Factors associated with mother-to-child transmission of hepatitis B virus despite immunoprophylaxis. Intern Med Tokyo Jpn. 2015;54(7):711-6. doi: https://doi.org/10.2169/internalmedicine.54.3514.
Wen WH, Chang MH, Zhao LL, Ni YH, Hsu HY, Wu JF, et al. Mother-to-infant transmission of hepatitis B virus infection: significance of maternal viral load and strategies for intervention. J Hepatol. 2013;59(1):24-30. doi: https://doi.org/10.1016/j.jhep.2013.02.015.
Lu LL, Chen BX, Wang J, Wang D, Ji Y, Yi HG, et al. Maternal transmission risk and antibody levels against hepatitis B virus e antigen in pregnant women. Int J Infect Dis Off Publ Int Soc Infect Dis. 2014;28:41-4. doi: https://doi.org/10.1016/j.ijid.2014.07.028.
Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat. 2012;19(2):18-25. doi: https://doi.org/10.1111/j.1365-2893.2011.01492.x.
Ott J, Stevens G, Wiersma S. The risk of perinatal hepatitis B virus transmission: hepatitis B e antigen (HBeAg) prevalence estimates for all world regions. BMC Infect Dis. 2012;12:131. doi: https://doi.org/10.1186/1471-2334-12-131.
Lee C, Gong Y, Brok J, Boxall E, Gluud C. Hepatitis B immunisation for newborn infants of hepatitis B surface antigen-positive mothers. Cochrane Database Syst Rev. 2006;(2):CD004790. doi: https://doi.org/10.1002/14651858.CD004790.pub2.
Beasley R, Hwang L, Lee G, Lan C, Roan C, Huang F, et al. Prevention of perinatally transmitted hepatitis B virus infections with hepatitis B immune globulin and hepatitis B vaccine. Lancet Lond Engl. 1983;2(8359):1099-102. doi: https://doi.org/10.1016/S0140-6736(83)90624-4.
Pan C, Duan ZP, Bhamidimarri K, Zou HB, Liang XF, Li J, et al. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2012;10(5):452-9. doi: https://doi.org/10.1016/j.cgh.2011.10.041.
Brown R, McMahon B, Lok A, Wong J, Ahmed A, Mouchli M, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: a systematic review and meta-analysis. Hepatol Baltim Md. 2016;63(1):319-33. doi: https://doi.org/10.1002/hep.28302.
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370-98. doi: 10.1016/j.jhep.2017.03.021.
Shao Z, Al Tibi M, Wakim-Fleming J. Update on viral hepatitis in pregnancy. Cleve Clin J Med. 2017;84(3):202-6. doi: https://doi.org/10.3949/ccjm.84a.15139.
Chang C, Aziz N, Poongkunran M, Javaid A, Trinh H, Lau D, et al. Serum alanine aminotransferase and hepatitis B DNA flares in pregnant and postpartum women with chronic hepatitis B. Am J Gastroenterol. 2016;111(10):1410-5. doi: https://doi.org/10.1038/ajg.2016.296.
Giles M, Visvanathan K, Lewin S, Bowden S, Locarnini S, Spelman T, et al. Clinical and virological predictors of hepatic flares in pregnant women with chronic hepatitis B. Gut. 2015;64(11):1810-5. doi: https://doi.org/10.1136/gutjnl-2014-308211.
Wu Q, Huang H, Sun X, Pan M, He Y, Tan S, et al. Telbivudine prevents vertical transmission of hepatitis B virus from women with high viral loads: a prospective long-term study. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2015;13(6):1170-6. doi: https://doi.org/10.1016/j.cgh.2014.08.043.
Pan C, Duan Z, Dai E, Zhang S, Han G, Wang Y, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med. 2016;374(24):2324-34. doi: https://doi.org/10.1056/NEJMoa1508660.
Deng M, Zhou X, Gao S, Yang SG, Wang B, Chen HZ, et al. The effects of telbivudine in late pregnancy to prevent intrauterine transmission of the hepatitis B virus: a systematic review and meta-analysis. Virol J. 2012;9:185. doi: https://doi.org/10.1186/1743-422X-9-185.
Sarkar M, Terrault N. Ending vertical transmission of hepatitis B: the third trimester intervention. Hepatol Baltim Md. 2014;60(2):448-51. doi: https://doi.org/10.1002/hep.27145.
Ayres A, Yuen L, Jackson K, Manoharan S, Glass A, Maley M, et al. Short duration of lamivudine for the prevention of hepatitis B virus transmission in pregnancy: lack of potency and selection of resistance mutations. J Viral Hepat. 2014;21(11):809-17. doi: https://doi.org/10.1111/jvh.12212.
Chen JZ, Liao ZW, Huang FL, Su RK, Wang WB, Cheng XY, et al. Efficacy and safety of tenofovir disoproxil fumarate in preventing vertical transmission of hepatitis B in pregnancies with high viral load. Sci Rep. 2017;7(1):4132. doi: https://doi.org/10.1038/s41598-017-04479-x.
Jourdain G, Ngo-Giang-Huong N, Cressey T, Hua L, Harrison L, Tierney C, et al. Prevention of mother-to-child transmission of hepatitis B virus: a phase III, placebo-controlled, double-blind, randomized clinical trial to assess the efficacy and safety of a short course of tenofovir disoproxil fumarate in women with hepatitis B virus e-antigen. BMC Infect Dis. 2016;16:393. doi: https://doi.org/10.1186/s12879-016-1734-5.
Hu Y, Chen J, Wen J, Xu C, Zhang S, Xu B, et al. Effect of elective cesarean section on the risk of mother-to-child transmission of hepatitis B virus. BMC Pregnancy Childbirth. 2013;13:119. doi: https://doi.org/10.1186/1471-2393-13-119.
Yang J, Zeng X, Men Y, Zhao L. Elective caesarean section versus vaginal delivery for preventing mother to child transmission of hepatitis B virus-A systematic review. Virol J. 2008;5:100. doi: https://doi.org/10.1186/1743-422X-5-100.
Hill J, Sheffield J, Kim M, Alexander J, Sercely B, Wendel G. Risk of hepatitis B transmission in breast-fed infants of chronic hepatitis B carriers. Obstet Gynecol. 2002;99(6):1049-52. doi: https://doi.org/10.1097/00006250-200206000-00018.
Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827-41. doi: https://doi.org/10.1542/peds.2011-3552.
Benaboud S, Pruvost A, Coffie P, Ekouévi D, Urien S, Arrivé E, et al. Concentrations of tenofovir and emtricitabine in breast milk of HIV-1-infected women in Abidjan, Cote d'Ivoire, in the ANRS 12109 TEmAA Study, Step 2. Antimicrob Agents Chemother. 2011;55(3):1315-7. doi: https://doi.org/10.1128/AAC.00514-10.
Brown R, Verna E, Pereira M, Tilson H, Aguilar C, Leu CS, et al. Hepatitis B virus and human immunodeficiency virus drugs in pregnancy: findings from the Antiretroviral Pregnancy Registry. J Hepatol. 2012;57(5):953-9. doi: https://doi.org/10.1016/j.jhep.2012.06.031.
Ayoub W, Cohen E. Hepatitis B management in the pregnant patient: an update. J Clin Transl Hepatol. 2016;4(3):241-7. doi: https://doi.org/10.14218/JCTH.2016.00014.
European association for the study of the liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69(2):461-511. doi: 10.1016/j.jhep.2018.03.026.
Page C, Hughes B, Rhee E, Kuller J. Hepatitis C in pregnancy: review of current knowledge and updated recommendations for management. Obstet Gynecol Surv. 2017;72(6):347-55. doi: https://doi.org/10.1097/OGX.0000000000000442.
Pott H, Theodoro M, de Almeida Vespoli J, Senise J, Castelo A. Mother-to-child transmission of hepatitis C virus. Eur J Obstet Gynecol Reprod Biol. 2018;224:125-30. doi: https://doi.org/10.1016/j.ejogrb.2018.03.034.
Benova L, Mohamoud Y, Calvert C, Abu-Raddad L. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014;59(6):765-73. doi: https://doi.org/10.1093/cid/ciu447.
Delotte J, Barjoan E, Berrébi A, Laffont C, Benos P, Pradier C, et al. Obstetric management does not influence vertical transmission of HCV infection: results of the ALHICE group study. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2014;27(7):664-70. doi: https://doi.org/10.3109/14767058.2013.829813.
Selvapatt N, Ward T, Bailey H, Bennett H, Thorne C, See LM, et al. Is antenatal screening for hepatitis C virus cost-effective? A decade's experience at a London centre. J Hepatol. 2015;63(4):797-804. doi: https://doi.org/10.1016/j.jhep.2015.05.015.
Diab-Elschahawi M, Dosch V, Honsig C, Jatzko B, Segagni L, Assadian O, et al. Evaluation of a universal vs a targeted hepatitis C virus screening strategy among pregnant women at the Vienna University Hospital. Am J Infect Control. 2013;41(5):459-60. doi: https://doi.org/10.1016/j.ajic.2012.06.003.
Bernstein H, Dunkelberg J, Leslie K. Hepatitis C in pregnancy in the era of direct-acting antiviral treatment: potential benefits of universal screening and antepartum therapy. Clin Obstet Gynecol. 2018;61(1):146-56. doi: https://doi.org/10.1097/GRF.0000000000000345.
Cottrell E, Chou R, Wasson N, Rahman B, Guise JM. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;158(2):109-13. doi: https://doi.org/10.7326/0003-4819-158-2-201301150-00575.
Valladares G, Chacaltana A, Sjogren MH. The management of HCV-infected pregnant women. Ann Hepatol. 2010;9:92-7. doi: https://doi.org/10.1016/S1665-2681(19)31731-4.
Barritt A, Jhaveri R. Treatment of hepatitis C during pregnancy-weighing the risks and benefits in contrast to HIV. Curr HIV/AIDS Rep. 2018;15(2):155-61. doi: https://doi.org/10.1007/s11904-018-0386-z.
Benjaminov F, Heathcote J. Liver disease in pregnancy. Am J Gastroenterol. 2004;99(12):2479-88. doi: 10.1111/j.1572-0241.2004.30231.x.
Giard JM, Terrault N. Women with cirrhosis. Gastroenterol Clin North Am. 2016;45(2):345-58. doi: https://doi.org/10.1016/j.gtc.2016.02.010.
Ramirez C, Doria C. Pregnancy after liver transplantation. Best Pract Res Clin Obstet Gynaecol. 2014;28(8):1137-45. doi: https://doi.org/10.1016/j.bpobgyn.2014.07.022.
Blume C, Pischke S, von Versen-Höynck F, Günter H, Gross M. Pregnancies in liver and kidney transplant recipients: a review of the current literature and recommendation. Best Pract Res Clin Obstet Gynaecol. 2014;28(8):1123-36. doi: https://doi.org/10.1016/j.bpobgyn.2014.07.021.
Akarsu M, Unek T, Avcu A, Ozbilgin M, Egeli T, Astarcioglu I. Evaluation of pregnancy outcomes after liver transplantation. Transplant Proc. 2016;48(10):3373-7. doi: https://doi.org/10.1016/j.transproceed.2016.09.033.

Downloads
Publicado
Como Citar
Edição
Seção
Licença
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
Los autores/as ceden sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los contenidos están protegidos bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.


| Métricas do artigo | |
|---|---|
| Vistas abstratas | |
| Visualizações da cozinha | |
| Visualizações de PDF | |
| Visualizações em HTML | |
| Outras visualizações | |