Microsatellite instability in Colombian patients with colorectal adenocarcinoma
DOI:
https://doi.org/10.22516/25007440.686Keywords:
Colon, Microsatelite instability, ColombiaAbstract
Introduction: The microsatellite instability (MSI) pathway is involved in the carcinogenesis of 15% of colorectal carcinomas (CRC). The detection of this alteration is relevant for the prognosis and treatment of CRC patients.
Objective: The aim of this study is to determine the prevalence of MSI in CRC in a cohort of patients in Bogotá, Colombia.
Materials and methods: The presence of MLH1, PMS2, MSH2, and MSH6 was evaluated by immunohistochemistry in CRC samples collected during colectomy. Clinicopathological variables were
analyzed as well. Cases with loss of MLH1 and PMS2 were evaluated for BRAF gene mutation.
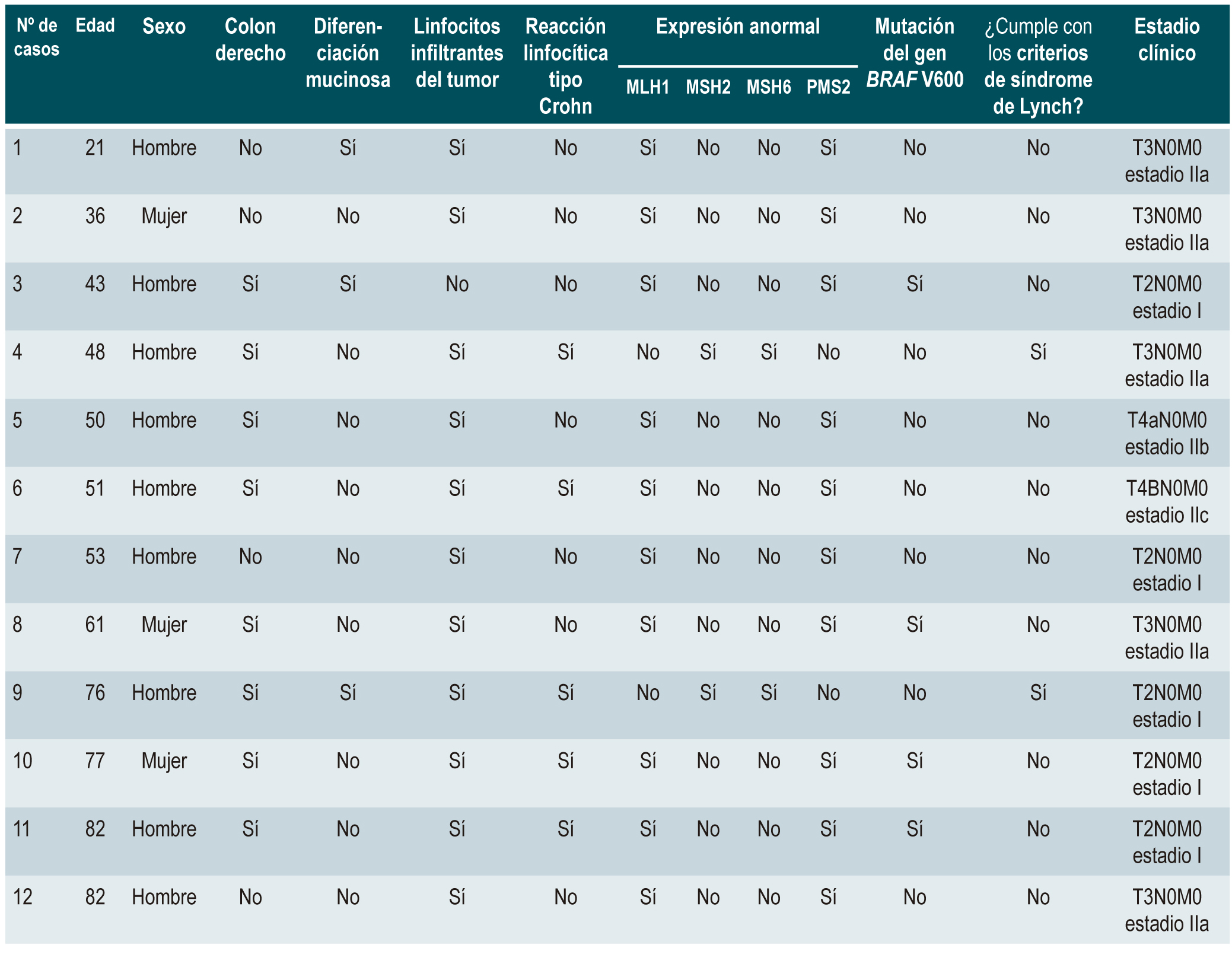
Results: A total of 86 cases were included. The median age was 69 years, 52.3% were male. 12 (13.9%) patients had IMS, 10 (83.3%) had absence of MLH1/PMS2 expression and 2 (16.7%) absence of MSH2/MSH6 expression. The median age of patients with IMS was 52 years (45-76.5), of which 9 were male. 66.7% of carcinomas were located in the right colon and the most frequent histological type was moderately differentiated adenocarcino(67%). Tumor infiltrating lymphocytes were observed in 83% of the cases, while the presence of Crohn’s-like infiltrate was present in 42%. BRAF mutation was observed in 30% of patients with loss of MLH1 and PMS2.
Conclusion: The prevalence of IMS in our population was 14%, similar to the data observed in the North American and European populations. However, we observed that 83% had loss of expression of the MLH1/PMS2 complex, a higher prevalence compared to other populations.
Downloads
References
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941-1953. https://doi.org/10.1002/ijc.31937
Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, Wolfe C, Hamadeh RR, Moore A, Werdecker A, Gessner BD, Te Ao B, McMahon B, Karimkhani C, Yu C, Cooke GS, Schwebel DC, Carpenter DO, Pereira DM, Nash D, Kazi DS, De Leo D, Plass D, Ukwaja KN, Thurston GD, Yun Jin K, Simard EP, Mills E, Park EK, Catalá-López F, deVeber G, Gotay C, Khan G, Hosgood HD 3rd, Santos IS, Leasher JL, Singh J, Leigh J, Jonas JB, Sanabria J, Beardsley J, Jacobsen KH, Takahashi K, Franklin RC, Ronfani L, Montico M, Naldi L, Tonelli M, Geleijnse J, Petzold M, Shrime MG, Younis M, Yonemoto N, Breitborde N, Yip P, Pourmalek F, Lotufo PA, Esteghamati A, Hankey GJ, Ali R, Lunevicius R, Malekzadeh R, Dellavalle R, Weintraub R, Lucas R, Hay R, Rojas-Rueda D, Westerman R, Sepanlou SG, Nolte S, Patten S, Weichenthal S, Abera SF, Fereshtehnejad SM, Shiue I, Driscoll T, Vasankari T, Alsharif U, Rahimi-Movaghar V, Vlassov VV, Marcenes WS, Mekonnen W, Melaku YA, Yano Y, Artaman A, Campos I, MacLachlan J, Mueller U, Kim D, Trillini M, Eshrati B, Williams HC, Shibuya K, Dandona R, Murthy K, Cowie B, Amare AT, Antonio CA, Castañeda-Orjuela C, van Gool CH, Violante F, Oh IH, Deribe K, Soreide K, Knibbs L, Kereselidze M, Green M, Cardenas R, Roy N, Tillmann T, Li Y, Krueger H, Monasta L, Dey S, Sheikhbahaei S, Hafezi-Nejad N, Kumar GA, Sreeramareddy CT, Dandona L, Wang H, Vollset SE, Mokdad A, Salomon JA, Lozano R, Vos T, Forouzanfar M, Lopez A, Murray C, Naghavi M. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1(4):505-27. https://doi.org/10.1001/jamaoncol.2015.0735
Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, French AJ, Goldberg RM, Hamilton SR, Laurent-Puig P, Gryfe R, Shepherd LE, Tu D, Redston M, Gallinger S. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247-57. https://doi.org/10.1056/NEJMoa022289
Xiao H, Yoon YS, Hong SM, Roh SA, Cho DH, Yu CS, Kim JC. Poorly differentiated colorectal cancers: correlation of microsatellite instability with clinicopathologic features and survival. Am J Clin Pathol. 2013;140(3):341-7.
https://doi.org/10.1309/AJCP8P2DYNKGRBVI
Slattery ML, Curtin K, Anderson K, Ma KN, Ballard L, Edwards S, Schaffer D, Potter J, Leppert M, Samowitz WS. Associations between cigarette smoking, lifestyle factors, and microsatellite instability in colon tumors. J Natl Cancer Inst. 2000;92(22):1831-6. https://doi.org/10.1093/jnci/92.22.1831
Liu D. Handbook of Tumor Syndromes. 1.a edición. CRC Press; 2020. https://doi.org/10.1201/9781351187435
Souglakos J. Genetic alterations in sporadic and hereditary colorectal cancer: implementations for screening and follow-up. Dig Dis. 2007;25(1):9-19. https://doi.org/10.1159/000099166
Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol. 2017;12(1):24. https://doi.org/10.1186/s13000-017-0613-8
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509-20. https://doi.org/10.1056/NEJMoa1500596
Tiwari AK, Roy HK, Lynch HT. Lynch syndrome in the 21st century: clinical perspectives. QJM. 2016;109(3):151-8. https://doi.org/10.1093/qjmed/hcv137
López-Correa PE, Lino-Silva LS, Gamboa-Domínguez A, Zepeda-Najar C, Salcedo-Hernández RA. Frequency of Defective Mismatch Repair System in a Series of Consecutive Cases of Colorectal Cancer in a National Cancer Center. J Gastrointest Cancer. 2018;49(3):379-384. https://doi.org/10.1007/s12029-018-0132-1
Leite SM, Gomes KB, Pardini VC, Ferreira AC, Oliveira VC, Cruz GM. Assessment of microsatellite instability in colorectal cancer patients from Brazil. Mol Biol Rep. 2010;37(1):375-80. https://doi.org/10.1007/s11033-009-9807-9
Egoavil CM, Montenegro P, Soto JL, Casanova L, Sanchez-Lihon J, Castillejo MI, Martinez-Canto A, Perez-Carbonell L, Castillejo A, Guarinos C, Barbera VM, Jover R, Paya A, Alenda C. Clinically important molecular features of Peruvian colorectal tumours: high prevalence of DNA mismatch repair deficiency and low incidence of KRAS mutations. Pathology. 2011;43(3):228-33. https://doi.org/10.1097/PAT.0b013e3283437613
Ortiz C, Dongo-Pflucker K, Martín-Cruz L, Barletta Carrillo C, Mora-Alferez P, Arias A. Inestabilidad de microsatélites en pacientes con diagnóstico de cáncer colorrectal. Rev Gastroenterol Perú. 2016;36(1):15-22.
Vaccaro CA, Carrozzo JE, Mocetti E, Berho M, Valdemoros P, Mullen E, Oviedo M, Redal MA. Expresión inmunohistoquímica e inestabilidad microsatelital en el síndrome de Lynch. Medicina (B Aires). 2007;67(3):274-8.
Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7(3):153-62. https://doi.org/10.1038/nrclinonc.2009.237
Baracaldo R, Peña L, Gómez O, Polo JF, López P, Parra-Medina R. Características histopatológicas del carcinoma colorrectal con inestabilidad microsatelital (IMS). Repert Med Cir. 2019;29(1):32-40. https://doi.org/10.31260/RepertMedCir.v29.n1.2020.172
Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. World Health Organization; 2010.
Giardiello FM, Allen JI, Axilbund JE, Boland CR, Burke CA, Burt RW, Church JM, Dominitz JA, Johnson DA, Kaltenbach T, Levin TR, Lieberman DA, Robertson DJ, Syngal S, Rex DK; US Multi-Society Task Force on Colorectal Cancer. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-Society Task Force on colorectal cancer. Gastroenterology. 2014;147(2):502-26. https://doi.org/10.1053/j.gastro.2014.04.001
Paredes SR, Chan C, Rickard MJFX. Immunohistochemistry in screening for heritable colorectal cancer: what to do with an abnormal result. ANZ J Surg. 2020;90(5):702-707. https://doi.org/10.1111/ans.15586
Parra-Medina R, Lopez-Correa P, Gutierrez V, Polo F. Colonic adenosquamous carcinoma and mucinous adenocarcinoma with microsatellite instability. Malays J Pathol. 2018;40(2):199-202.
Satia JA, Keku T, Galanko JA, Martin C, Doctolero RT, Tajima A, Sandler RS, Carethers JM. Diet, lifestyle, and genomic instability in the North Carolina Colon Cancer Study. Cancer Epidemiol Biomarkers Prev. 2005;14(2):429-36. https://doi.org/10.1158/1055-9965.EPI-04-0486
Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, Nakagawa H, Sotamaa K, Prior TW, Westman J, Panescu J, Fix D, Lockman J, Comeras I, de la Chapelle A. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med. 2005;352(18):1851-60. https://doi.org/10.1056/NEJMoa043146
Buchanan DD, Clendenning M, Rosty C, Eriksen SV, Walsh MD, Walters RJ, Thibodeau SN, Stewart J, Preston S, Win AK, Flander L, Ouakrim DA, Macrae FA, Boussioutas A, Winship IM, Giles GG, Hopper JL, Southey MC, English D, Jenkins MA. Tumor testing to identify lynch syndrome in two Australian colorectal cancer cohorts. J Gastroenterol Hepatol. 2017;32(2):427-438. https://doi.org/10.1111/jgh.13468
Alqahtani M, Grieu F, Carrello A, Amanuel B, Mashour M, Alattas R, Alsaleh K, Alsheikh A, Alqahtani S, Iacopetta B. Screening for Lynch Syndrome in Young Colorectal Cancer Patients from Saudi Arabia Using Microsatellite Instability as the Initial Test. Asian Pac J Cancer Prev. 2016;17(4):1917-23. https://doi.org/10.7314/apjcp.2016.17.4.1917
Gelsomino F, Barbolini M, Spallanzani A, Pugliese G, Cascinu S. The evolving role of microsatellite instability in colorectal cancer: A review. Cancer Treat Rev. 2016;51:19-26. https://doi.org/10.1016/j.ctrv.2016.10.005
Gupta S, Ashfaq R, Kapur P, Afonso BB, Nguyen TP, Ansari F, Boland CR, Goel A, Rockey DC. Microsatellite instability among individuals of Hispanic origin with colorectal cancer. Cancer. 2010;116(21):4965-72. https://doi.org/10.1002/cncr.25486
Shamekh R, Cives M, Mejia J, Coppola D. Higher frequency of isolated PMS2 loss in colorectal tumors in Colombian population: preliminary results. Pathology and Laboratory Medicine International. 2016;8:37-41. https://doi.org/10.2147/PLMI.S94771
Montenegro M Y, Ramírez-Castro JL, Isaza J LF, Bedoya B G, Muñetón-Peña CM. Análisis genético en pacientes con cáncer colorrectal. Revista Médica de Chile. 2006;134(10):1221-9. https://doi.org/10.4067/S0034-98872006001000002
Cárdenas W CA, Vargas C, Moreno O, Insuasti J. Análisis de la inestabilidad de microsatélites mediante el marcador BAT-26 en una muestra de pacientes del Hospital Universitario de Santander con diagnóstico de cáncer gástrico o colorrectal. Colombia Médica. 2008;39(Supl 2):41-51.
Afanador Ayala CH, Palacio Rúa KA, Isaza Jiménez LF, Ahumada Rodríguez E, Ocampo CM, Muñetón Peña CM. Análisis de inestabilidad microsatelital en individuos con cáncer colorrectal esporádico del departamento de Antioquia. Revista Colombiana de Cancerología. 2017;21(1):51-2. https://doi.org/10.1016/j.rccan.2017.02.017
Terdiman JP. It is time to get serious about diagnosing Lynch syndrome (hereditary nonpolyposis colorectal cancer with defective DNA mismatch repair) in the general population. Gastroenterology. 2005;129(2):741-4. https://doi.org/10.1016/j.gastro.2005.06.033
Ashktorab H, Smoot DT, Carethers JM, Rahmanian M, Kittles R, Vosganian G, Doura M, Nidhiry E, Naab T, Momen B, Shakhani S, Giardiello FM. High incidence of microsatellite instability in colorectal cancer from African Americans. Clin Cancer Res. 2003;9(3):1112-7.
Kakar S, Shi C, Berho ME, Driman DK, Fitzgibbons P, Frankel WL, Hill KA, Jessup J, Krasinskas AMN, Washington MK. Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Colon and Rectum [internet]. College of American Pathologists; 2017 [consultado el 15de junio de 2020]. Disponible en: https://documents.cap.org/protocols/cp-gilower-colonrectum-17protocol-4010.pdf
Downloads
Published
How to Cite
Issue
Section
License
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
Los autores/as ceden sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los contenidos están protegidos bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.


| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |














