Consenso colombiano de la enfermedad inflamatoria intestinal pediátrica
DOI:
https://doi.org/10.22516/25007440.943Palabras clave:
Enfermedad inflamatoria intestinal, Enfermedad de Crohn, Colitis ulcerativa, PediatríaResumen
Introducción: la colitis ulcerativa pediátrica (CUP), la enfermedad de Crohn pediátrica (ECP) y la enfermedad inflamatoria intestinal pediátrica no clasificable (EIIPNC) tienen particularidades clínicas y psicosociales que las diferencian de las del adulto y pueden condicionar enfoques terapéuticos distintos por las posibles repercusiones nutricionales, crecimiento y desarrollo, lo que representa un desafío para el pediatra y el gastroenterólogo.
Objetivo: desarrollar recomendaciones basadas en la evidencia por consenso de expertos para el diagnóstico y el tratamiento oportunos y seguros de la enfermedad inflamatoria intestinal pediátrica (EIIP) en menores de 18 años, para los profesionales que atienden estos pacientes y los pagadores en salud.
Metodología: a través de un panel de expertos del Colegio Colombiano de Gastroenterología, Hepatología y Nutrición Pediátrica (COLGAHNP) y un grupo multidisciplinario se formularon 35 preguntas en relación con el cuadro clínico, el diagnóstico y el tratamiento de la EIIP. A través de una revisión y un análisis crítico de la literatura, con especial énfasis en las principales guías de práctica clínica (GPC), estudios clínicos aleatorizados (ECA) y metaanálisis de los últimos 10 años, los expertos plantearon 77 recomendaciones que respondían a cada una de las preguntas de investigación con sus respectivos puntos prácticos. Posteriormente, cada una de las afirmaciones se sometieron a votación dentro del grupo desarrollador, incluyendo las afirmaciones que alcanzaron > 80 %.
Resultados: todas las afirmaciones alcanzaron una votación > 80 %. La EIIP tiene mayor extensión, severidad y evolución hacia la estenosis, enfermedad perianal, manifestaciones extraintestinales y retraso en el crecimiento en comparación con los pacientes adultos, por lo que su manejo debe ser realizado por grupos multidisciplinarios liderados por gastroenterólogos pediatras y prepararlos para una transición a la edad adulta. Los criterios de Porto permiten una clasificación práctica de la EIIP. En la ECP, debemos usar la clasificación de París y debemos realizar ileocolonoscopia y esofagogastroduodenoscopia, ya que el 50 % tienen un compromiso superior, usando el SES-CD (UCEIS/Mayo en CUP) y tomando múltiples biopsias. Los laboratorios iniciales deben incluir marcadores de inflamación, calprotectina fecal y descartar infecciones intestinales. El tratamiento, la inducción y el mantenimiento de la EIIP deben ser individualizados y decididos según la estratificación de riesgo. En el seguimiento se debe usar el Pediatric Crohn Disease Activity Index (PCDAI) y Pediatric Ulcerative Colitis Activity Index (PUCAI) de las últimas 48 horas. Los pacientes con EIIP temprana e infantil, deben ser valorados por inmunólogos y genetistas.
Conclusión: se proporciona una guía de consenso con recomendaciones basadas en la evidencia sobre el diagnóstico y los tratamientos oportunos y seguros en los pacientes con EIIP.
Descargas
Referencias bibliográficas
Roberts SE, Thorne K, Thapar N, Broekaert I, Benninga MA, Dolinsek J, Mas E, Miele E, Orel R, Pienar C, Ribes-Koninckx C, Thomson M, Tzivinikos C, Morrison-Rees S, John A, Williams JG. A Systematic Review and Meta-analysis of Paediatric Inflammatory Bowel Disease Incidence and Prevalence Across Europe. J Crohns Colitis. 2020;14(8):1119-48. https://doi.org/10.1093/ecco-jcc/jjaa037
Saeed SA, Kugathasan S. Epidemiology of Pediatric Inflammatory Bowel Disease. En: Mamula P, Grossman AB, Baldassano RN, Kelsen JR, Markowitz JE, editores. Pediatric Inflammatory Bowel Disease. Cham: Springer International Publishing; 2017. p. 71-86.
Larrosa-Haro A, Abundis-Castro L, Contreras MB, Gallo MJ, Peña-Quintana L, Targa Ferreira CH, Nacif PA, Vázquez-Frias R, Bravo S, Muñoz-Urribarri AB, Mejía-Castro M, Orsi M, Amil-Díaz J, Busoni V, Cohen-Sabban J, Martin-Capri FJ, Zablah R, Rodríguez-Guerrero MG, Sdepanian VL. Tendencia epidemiológica de la enfermedad intestinal inflamatoria en pacientes pediátricos en América Latina: Grupo de Trabajo en Enfermedad Intestinal Inflamatoria, Sociedad Latinoamericana de Gastroenterología, Hepatología y Nutrición Pediátrica (SLAGHNP). Rev Gastroenterol México. 2021;86(4):328-34. https://doi.org/10.1016/j.rgmx.2020.07.010
Gonzalez M, Ossa JC, Alliende F, Canales P, Cofré C, Faúndez R. Enfermedad Inflamatoria Intestinal en pediatría (EII): revisión. Grupo de trabajo de la Sociedad Latinoamericana de Gastroenterología, Hepatología y Nutrición Pediátrica (SLAGHNP). Acta Gastroenterol Latinoam. 2007;48(3):299-307.
Birimberg-Schwartz L, Zucker DM, Akriv A, Cucchiara S, Cameron FL, Wilson DC, Lazowska I, Yianni L, Paul SP, Romano C, Kolacek S, Buderus S, Pærregaard A, Russell RK, Escher JC, Turner D; Pediatric IBD Porto group of ESPGHAN. Development and Validation of Diagnostic Criteria for IBD Subtypes Including IBD-unclassified in Children: a Multicentre Study From the Pediatric IBD Porto Group of ESPGHAN. J Crohns Colitis. 2017;11(9):1078-084. https://doi.org/10.1093/ecco-jcc/jjx053
Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, Kolho KL, Veres G, Russell RK, Paerregaard A, Buderus S, Greer ML, Dias JA, Veereman-Wauters G, Lionetti P, Sladek M, Martin de Carpi J, Staiano A, Ruemmele FM, Wilson DC; European Society of Pediatric Gastroenterology, Hepatology, and Nutrition.ESPGHAN Revised Porto Criteria for the Diagnosis of Inflammatory Bowel Disease in Children and Adolescents. J Pediatr Gastroenterol Nutr. 2014;58(6):795-806. https://doi.org/10.1097/MPG.0000000000000239
Amil-Dias J, Kolacek S, Turner D, Pærregaard A, Rintala R, Afzal NA, Karolewska-Bochenek K, Bronsky J, Chong S, Fell J, Hojsak I, Hugot JP, Koletzko S, Kumar D, Lazowska-Przeorek I, Lillehei C, Lionetti P, Martin-de-Carpi J, Pakarinen M, Ruemmele FM, Shaoul R, Spray C, Staiano A, Sugarman I, Wilson DC, Winter H, Kolho KL; IBD Working Group of ESPGHAN (IBD Porto Group). Surgical Management of Crohn Disease in Children: Guidelines From the Paediatric IBD Porto Group of ESPGHAN. J Pediatr Gastroenterol Nutr. 2017;64(5):818-35. https://doi.org/10.1097/MPG.0000000000001562
Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, Amil Dias J, Barabino A, Braegger CP, Bronsky J, Buderus S, Martín-de-Carpi J, De Ridder L, Fagerberg UL, Hugot JP, Kierkus J, Kolacek S, Koletzko S, Lionetti P, Miele E, Navas López VM, Paerregaard A, Russell RK, Serban DE, Shaoul R, Van Rheenen P, Veereman G, Weiss B, Wilson D, Dignass A, Eliakim A, Winter H, Turner D; European Crohn’s and Colitis Organisation; European Society of Pediatric Gastroenterology, Hepatology and Nutrition. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014;8(10):1179-207. https://doi.org/10.1016/j.crohns.2014.04.005
Turner D, Levine A, Escher JC, Griffiths AM, Russell RK, Dignass A, Dias JA, Bronsky J, Braegger CP, Cucchiara S, de Ridder L, Fagerberg UL, Hussey S, Hugot JP, Kolacek S, Kolho KL, Lionetti P, Paerregaard A, Potapov A, Rintala R, Serban DE, Staiano A, Sweeny B, Veerman G, Veres G, Wilson DC, Ruemmele FM; European Crohn’s and Colitis Organization; European Society for Paediatric Gastroenterology, Hepatology, and Nutrition. Management of Pediatric Ulcerative Colitis: Joint ECCO and ESPGHAN Evidence-based Consensus Guidelines. J Pediatr Gastroenterol Nutr. 2012;55(3):340-61. https://doi.org/10.1097/MPG.0b013e3182662233
Turner D, Ruemmele FM, Orlanski-Meyer E, Griffiths AM, de Carpi JM, Bronsky J, et al. Management of Paediatric Ulcerative Colitis, Part 1: Ambulatory Care-An Evidence-based Guideline From European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;67(2):257-91. https://doi.org/10.1097/MPG.0000000000002035
Turner D, Ruemmele FM, Orlanski-Meyer E, Griffiths AM, de Carpi JM, Bronsky J, Veres G, Aloi M, Strisciuglio C, Braegger CP, Assa A, Romano C, Hussey S, Stanton M, Pakarinen M, de Ridder L, Katsanos KH, Croft N, Navas-López VM, Wilson DC, Lawrence S, Russell RK. Management of Paediatric Ulcerative Colitis, Part 2: Acute Severe Colitis-An Evidence-based Consensus Guideline From the European Crohn’s and Colitis Organization and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;67(2):292-310. https://doi.org/10.1097/MPG.0000000000002036
Turner D, Travis SP, Griffiths AM, Ruemmele FM, Levine A, Benchimol EI, Dubinsky M, Alex G, Baldassano RN, Langer JC, Shamberger R, Hyams JS, Cucchiara S, Bousvaros A, Escher JC, Markowitz J, Wilson DC, van Assche G, Russell RK; European Crohn’s and Colitis Organization; Porto IBD Working Group, European Society of Pediatric Gastroenterology, Hepatology, and Nutrition. Consensus for Managing Acute Severe Ulcerative Colitis in Children: A Systematic Review and Joint Statement From ECCO, ESPGHAN, and the Porto IBD Working Group of ESPGHAN. Am J Gastroenterol. 2011;106(4):574-88. https://doi.org/10.1038/ajg.2010.481
Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, Fell J, Ruemmele FM, Walters T, Sherlock M, Dubinsky M, Hyams JS. Pediatric modification of the Montreal classification for inflammatory bowel disease: The Paris classification. Inflamm Bowel Dis. 2011;17(6):1314-21. https://doi.org/10.1002/ibd.21493
Forbes A, Escher J, Hébuterne X, Kłęk S, Krznaric Z, Schneider S, Shamir R, Stardelova K, Wierdsma N, Wiskin AE, Bischoff SC. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin Nutr. 2017;36(2):321-47. https://doi.org/10.1016/j.clnu.2016.12.027
Bischoff SC, Escher J, Hébuterne X, Kłęk S, Krznaric Z, Schneider S, Shamir R, Stardelova K, Wierdsma N, Wiskin AE, Forbes A. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin Nutr. 2020;39(3):632-53. https://doi.org/10.1016/j.clnu.2019.11.002
Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, Atkins D; Methods Work Group, Third US Preventive Services Task Force. Current methods of the U.S. Preventive Services Task Force. Am J Prev Med. 2001;20(3):21-35. https://doi.org/10.1016/S0749-3797(01)00261-6
Sawaya GF, Guirguis-Blake J, LeFevre M, Harris R, Petitti D; U.S. Preventive Services Task Force. Update on the Methods of the U.S. Preventive Services Task Force: Estimating Certainty and Magnitude of Net Benefit. Ann Intern Med. 2007;147(12):871-5. https://doi.org/10.7326/0003-4819-147-12-200712180-00007
Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique: Delphi survey technique. J Adv Nurs. 2000;32(4):1008-15. https://doi.org/10.1046/j.1365-2648.2000.t01-1-01567.x
Varela-Ruiz M, Díaz-Bravo L, García-Durán R. Descripción y usos del método Delphi en investigaciones del área de la salud. Investig En Educ Médica. 2012;1(2):90-5.
Guariso G, Gasparetto M, Visonà Dalla Pozza L, D’Incà R, Zancan L, Sturniolo G, Brotto F, Facchin P. Inflammatory Bowel Disease Developing in Paediatric and Adult Age. J Pediatr Gastroenterol Nutr. 2010;51(6):698-707. https://doi.org/10.1097/MPG.0b013e3181da1db8
Sonavane AD, Sonawane P, Amarapurkar DN. Inflammatory Bowel Disease Across the Age Continuum: Similarity and Disparity. Indian J Pediatr. 2018;85(11):989-94. https://doi.org/10.1007/s12098-018-2665-5
Yu YR, Rodriguez JR. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin Pediatr Surg. 2017;26(6):349-55. https://doi.org/10.1053/j.sempedsurg.2017.10.003
Eszter Müller K, Laszlo Lakatos P, Papp M, Veres G. Incidence and Paris Classification of Pediatric Inflammatory Bowel Disease. Gastroenterol Res Pract. 2014;2014: 904307. https://doi.org/10.1155/2014/904307
Oliva S, Thomson M, de Ridder L, Martín-de-Carpi J, Van Biervliet S, Braegger C, Dias JA, Kolacek S, Miele E, Buderus S, Bronsky J, Winter H, Navas-López VM, Assa A, Chong SKF, Afzal NA, Smets F, Shaoul R, Hussey S, Turner D, Cucchiara S. Endoscopy in Pediatric Inflammatory Bowel Disease: A Position Paper on Behalf of the Porto IBD Group of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;67(3):414-30. https://doi.org/10.1097/MPG.0000000000002092
De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, Liew D, Prideaux L, Lawrance IC, Andrews JM, Bampton PA, Gibson PR, Sparrow M, Leong RW, Florin TH, Gearry RB, Radford-Smith G, Macrae FA, Debinski H, Selby W, Kronborg I, Johnston MJ, Woods R, Elliott PR, Bell SJ, Brown SJ, Connell WR, Desmond PV. Crohn’s disease management after intestinal resection: a randomised trial. The Lancet. 2015;385(9976):1406-17. https://doi.org/10.1016/S0140-6736(14)61908-5
Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, Troncone R, Giersiepen K, Branski D, Catassi C, Lelgeman M, Mäki M, Ribes-Koninckx C, Ventura A, Zimmer KP; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastroenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition Guidelines for the Diagnosis of Coeliac Disease. J Pediatr Gastroenterol Nutr. 2012;54(1):136-60. https://doi.org/10.1097/MPG.0b013e31821a23d0
de Bie CI, Buderus S, Sandhu BK, de Ridder L, Paerregaard A, Veres G, Dias JA, Escher JC; EUROKIDS Porto IBD Working Group of ESPGHAN. Diagnostic Workup of Paediatric Patients With Inflammatory Bowel Disease in Europe: Results of a 5-Year Audit of the EUROKIDS Registry. J Pediatr Gastroenterol Nutr. 2012;54(3):374-80. https://doi.org/10.1097/MPG.0b013e318231d984
Neville JJ, Macdonald A, Fell J, Choudhry M, Haddad M. Therapeutic strategies for stricturing Crohn’s disease in childhood: a systematic review. Pediatr Surg Int. 2021;37(5):569-77. https://doi.org/10.1007/s00383-020-04848-0
Khanna R, Bouguen G, Feagan BG, D’Haens G, Sandborn WJ, Dubcenco E, Baker KA, Levesque BG. A Systematic Review of Measurement of Endoscopic Disease Activity and Mucosal Healing in Crohn’s Disease: Recommendations for Clinical Trial Design. Inflamm Bowel Dis. 2014;20(10):1850-61. https://doi.org/10.1097/MIB.0000000000000131
Walsh A, Palmer R, Travis S. Mucosal Healing As a Target of Therapy for Colonic Inflammatory Bowel Disease and Methods to Score Disease Activity. Gastrointest Endosc Clin N Am. 2014;24(3):367-78. https://doi.org/10.1016/j.giec.2014.03.005
Fuller MK. Pediatric Inflammatory Bowel Disease. Surg Clin North Am. 2019;99(6):1177-83. https://doi.org/10.1016/j.suc.2019.08.008
Dalzell AM, Ba’Ath ME. Paediatric inflammatory bowel disease: review with a focus on practice in low- to middle-income countries. Paediatr Int Child Health. 2019;39(1):48-58. https://doi.org/10.1080/20469047.2019.1575056
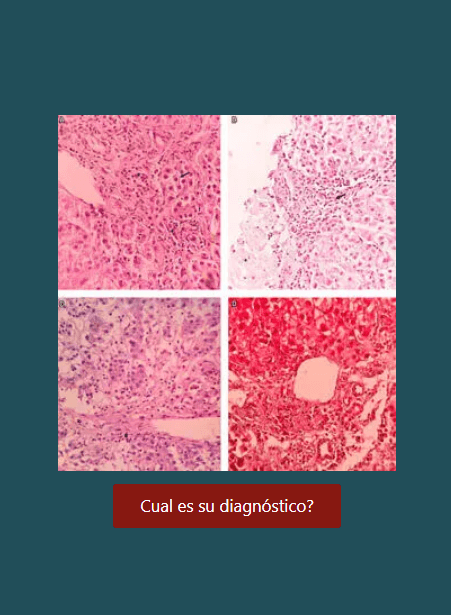
Magro F, Langner C, Driessen A, Ensari A, Geboes K, Mantzaris GJ, Villanacci V, Becheanu G, Borralho Nunes P, Cathomas G, Fries W, Jouret-Mourin A, Mescoli C, de Petris G, Rubio CA, Shepherd NA, Vieth M, Eliakim R; European Society of Pathology (ESP); European Crohn’s and Colitis Organisation (ECCO). European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. 2013;7(10):827-51. https://doi.org/10.1016/j.crohns.2013.06.001
Abuquteish D, Putra J. Upper gastrointestinal tract involvement of pediatric inflammatory bowel disease: A pathological review. World J Gastroenterol. 2019;25(16):1928-35. https://doi.org/10.3748/wjg.v25.i16.1928
Oliveira SB, Monteiro IM. Diagnosis and management of inflammatory bowel disease in children. BMJ. 2017;357:j2083. https://doi.org/10.1136/bmj.j2083
Däbritz J, Gerner P, Enninger A, Claßen M, Radke M. Inflammatory Bowel Disease in Childhood and Adolescence. Dtsch Arztebl Int. 2017;114(19):331-8. https://doi.org/10.3238/arztebl.2017.0331
Lemberg DA, Clarkson CM, Bohane TD, Day AS. Role of esophagogastroduodenoscopy in the initial assessment of children with inflammatory bowel disease. J Gastroenterol Hepatol. 2005;20(11):1696-700. https://doi.org/10.1111/j.1440-1746.2005.03954.x
Battat R, Vande Casteele N, Pai RK, Wang Z, Zou G, McDonald JWD, Duijvestein M, Jeyarajah J, Parker CE, Van Viegen T, Nelson SA, Boland BS, Singh S, Dulai PS, Valasek MA, Feagan BG, Jairath V, Sandborn WJ. Evaluating the optimum number of biopsies to assess histological inflammation in ulcerative colitis: a retrospective cohort study. Aliment Pharmacol Ther. 2020;52(10):1574-582. https://doi.org/10.1111/apt.16083
Langner C, Magro F, Driessen A, Ensari A, Mantzaris GJ, Villanacci V, Becheanu G, Borralho Nunes P, Cathomas G, Fries W, Jouret-Mourin A, Mescoli C, de Petris G, Rubio CA, Shepherd NA, Vieth M, Eliakim R, Geboes K; European Society of Pathology; European Crohn’s and Colitis Foundation. The histopathological approach to inflammatory bowel disease: a practice guide. Virchows Arch. 2014;464(5):511-27. https://doi.org/10.1007/s00428-014-1543-4
Kellermann L, Riis LB. A close view on histopathological changes in inflammatory bowel disease, a narrative review. Dig Med Res. 2021;4:3. https://doi.org/10.21037/dmr-21-1
North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition; Colitis Foundation of America; Bousvaros A, Antonioli DA, Colletti RB, Dubinsky MC, Glickman JN, Gold BD, Griffiths AM, Jevon GP, Higuchi LM, Hyams JS, Kirschner BS, Kugathasan S, Baldassano RN, Russo PA. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J Pediatr Gastroenterol Nutr. 2007;44(5):653-74. https://doi.org/10.1097/MPG.0b013e31805563f3
Dubinsky M. Special issues in pediatric inflammatory bowel disease. World J Gastroenterol. 2007;14(3):413. https://doi.org/10.3748/wjg.14.413
Schreiber-Dietrich D, Chiorean L, Cui XW, Braden B, Kucharzik T, Jüngert J, Kosiak W, Stenzel M, Dietrich CF. Particularities of Crohn’s disease in pediatric patients: current status and perspectives regarding imaging modalities. Expert Rev Gastroenterol Hepatol. 2015;9(10):1313-25. https://doi.org/10.1586/17474124.2015.1083420
Allocca M, Danese S, Laurent V, Peyrin-Biroulet L. Use of Cross-Sectional Imaging for Tight Monitoring of Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2020;18(6):1309-1323.e4. https://doi.org/10.1016/j.cgh.2019.11.052
Rimola J, Rodriguez S, García-Bosch O, Ordás I, Ayala E, Aceituno M, Pellisé M, Ayuso C, Ricart E, Donoso L, Panés J. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut. 2009;58(8):1113-20. https://doi.org/10.1136/gut.2008.167957
Schooler GR, Hull NC, Mavis A, Lee EY. MR Imaging Evaluation of Inflammatory Bowel Disease in Children: Magn Reson Imaging Clin N Am. 2019;27(2):291-300. https://doi.org/10.1016/j.mric.2019.01.007
Maltz R, Podberesky DJ, Saeed SA. Imaging modalities in pediatric inflammatory bowel disease. Curr Opin Pediatr. 2014;26(5):590-6. https://doi.org/10.1097/MOP.0000000000000131
Pouillon L, Laurent V, Pouillon M, Bossuyt P, Bonifacio C, Danese S, Deepak P, Loftus EV Jr, Bruining DH, Peyrin-Biroulet L. Diffusion-weighted MRI in inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2018;3(6):433-43. https://doi.org/10.1016/S2468-1253(18)30054-2
Ordás I, Rimola J, Alfaro I, Rodríguez S, Castro-Poceiro J, Ramírez-Morros A, Gallego M, Giner À, Barastegui R, Fernández-Clotet A, Masamunt M, Ricart E, Panés J. Development and Validation of a Simplified Magnetic Resonance Index of Activity for Crohn’s Disease. Gastroenterology. 2019;157(2):432-439.e1. https://doi.org/10.1053/j.gastro.2019.03.051
Church PC, Greer MC, Cytter-Kuint R, Doria AS, Griffiths AM, Turner D, Walters TD, Feldman BM. Magnetic resonance enterography has good inter-rater agreement and diagnostic accuracy for detecting inflammation in pediatric Crohn disease. Pediatr Radiol. 2017;47(5):565-75. https://doi.org/10.1007/s00247-017-3790-4
Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T, Rieder F, Almer S, Armuzzi A, Harbord M, Langhorst J, Sans M, Chowers Y, Fiorino G, Juillerat P, Mantzaris GJ, Rizzello F, Vavricka S, Gionchetti P; ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis. 2017;11(1):3-25. https://doi.org/10.1093/ecco-jcc/jjw168
Koplay M, Guneyli S, Cebeci H, Korkmaz H, Emiroglu HH, Sekmenli T, Paksoy Y. Magnetic resonance enterography with oral mannitol solution: Diagnostic efficacy and image quality in Crohn disease. Diagn Interv Imaging. 2017;98(12):893-9. https://doi.org/10.1016/j.diii.2017.06.011
Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol. 2017;16(7):564-70. https://doi.org/10.1016/S1474-4422(17)30158-8
Kim DH, Carucci LR, Baker ME, Cash BD, Dillman JR, Feig BW, Fowler KJ, Gage KL, Noto RB, Smith MP, Yaghmai V, Yee J, Lalani T. ACR Appropriateness Criteria Crohn Disease. J Am Coll Radiol. 2015;12(10):1048-1057.e4. https://doi.org/10.1016/j.jacr.2015.07.005
Calabrese E, Kucharzik T, Maaser C, Maconi G, Strobel D, Wilson SR, Zorzi F, Novak KL, Bruining DH, Iacucci M, Watanabe M, Lolli E, Chiaramonte C, Hanauer SB, Panaccione R, Pallone F, Ghosh S, Monteleone G. Real-time Interobserver Agreement in Bowel Ultrasonography for Diagnostic Assessment in Patients With Crohn’s Disease: An International Multicenter Study. Inflamm Bowel Dis. 2018;24(9):2001-6. https://doi.org/10.1093/ibd/izy091
Calabrese E, Maaser C, Zorzi F, Kannengiesser K, Hanauer SB, Bruining DH, Iacucci M, Maconi G, Novak KL, Panaccione R, Strobel D, Wilson SR, Watanabe M, Pallone F, Ghosh S. Bowel Ultrasonography in the Management of Crohn’s Disease. A Review with Recommendations of an International Panel of Experts: Inflamm Bowel Dis. 2016;22(5):1168-83. https://doi.org/10.1097/MIB.0000000000000706
Chiorean L. Ultrasonographic imaging of inflammatory bowel disease in pediatric patients. World J Gastroenterol. 2015;21(17):5231. https://doi.org/10.3748/wjg.v21.i17.5231
Pallotta N, Civitelli F, Di Nardo G, Vincoli G, Aloi M, Viola F, Capocaccia P, Corazziari E, Cucchiara S. Small Intestine Contrast Ultrasonography in Pediatric Crohn’s Disease. J Pediatr. 2013;163(3):778-784.e1. https://doi.org/10.1016/j.jpeds.2013.03.056
Moreno N, Ripollés T, Paredes JM, Ortiz I, Martínez MJ, López A, Delgado F, Moreno-Osset E. Usefulness of abdominal ultrasonography in the analysis of endoscopic activity in patients with Crohn’s disease: Changes following treatment with immunomodulators and/or anti-TNF antibodies. J Crohns Colitis. 2014;8(9):1079-87. https://doi.org/10.1016/j.crohns.2014.02.008
Anupindi SA, Podberesky DJ, Towbin AJ, Courtier J, Gee MS, Darge K, Dillman JR. Pediatric inflammatory bowel disease: imaging issues with targeted solutions. Abdom Imaging. 2015;40(5):975-92. https://doi.org/10.1007/s00261-015-0423-y
Puylaert CAJ, Tielbeek JAW, Bipat S, Stoker J. Grading of Crohn’s disease activity using CT, MRI, US and scintigraphy: a meta-analysis. Eur Radiol. 2015;25(11):3295-313. https://doi.org/10.1007/s00330-015-3737-9.
Brodersen JB, Hess S. FDG-PET/CT in Inflammatory Bowel Disease. PET Clin. 2020;15(2):153-62. https://doi.org/10.1016/j.cpet.2019.11.006
Saade C, Nasr L, Sharara A, Barada K, Soweid A, Murad F, Tawil A, Ghieh D, Asmar K, Tamim H, Khoury NJ. Crohn’s disease: A retrospective analysis between computed tomography enterography, colonoscopy, and histopathology. Radiography. 2019;25(4):349-58. https://doi.org/10.1016/j.radi.2019.04.007
Heuschkel RB, Menache CC, Megerian JT, Baird AE. Enteral Nutrition and Corticosteroids in the Treatment of Acute Crohn’s Disease in Children: J Pediatr Gastroenterol Nutr. 2000;31(1):8-15. https://doi.org/10.1097/00005176-200007000-00005
Turner D, Levine A, Walters TD, Focht G, Otley A, López VN, Koletzko S, Baldassano R, Mack D, Hyams J, Griffiths AM. Which PCDAI Version Best Reflects Intestinal Inflammation in Pediatric Crohn Disease? J Pediatr Gastroenterol Nutr. 2017;64(2):254-60. https://doi.org/10.1097/MPG.0000000000001227
Grant A, Lerer T, Griffiths AM, Hyams J, Otley A. Assessing disease activity using the pediatric Crohn’s disease activity index: Can we use subjective or objective parameters alone? World J Gastroenterol. 2021;27(30):5100-11. https://doi.org/10.3748/wjg.v27.i30.5100
Henderson P, Anderson NH, Wilson DC. The Diagnostic Accuracy of Fecal Calprotectin During the Investigation of Suspected Pediatric Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2014;109(5):637-45. https://doi.org/10.1038/ajg.2013.131
van Rheenen PF, Aloi M, Assa A, Bronsky J, Escher JC, Fagerberg UL, Gasparetto M, Gerasimidis K, Griffiths A, Henderson P, Koletzko S, Kolho KL, Levine A, van Limbergen J, Martin de Carpi FJ, Navas-López VM, Oliva S, de Ridder L, Russell RK, Shouval D, Spinelli A, Turner D, Wilson D, Wine E, Ruemmele FM. The Medical Management of Paediatric Crohn’s Disease: an ECCO-ESPGHAN Guideline Update. J Crohns Colitis. 2021;15(2):171-94. https://doi.org/10.1093/ecco-jcc/jjaa161
Cozijnsen MA, Ben Shoham A, Kang B, Choe BH, Choe YH, Jongsma MME, Russell RK, Ruemmele FM, Escher JC, de Ridder L, Koletzko S, Martín-de-Carpi J, Hyams J, Walters T, Griffiths A, Turner D. Development and Validation of the Mucosal Inflammation Noninvasive Index For Pediatric Crohn’s Disease. Clin Gastroenterol Hepatol. 2020;18(1):133-140.e1. https://doi.org/10.1016/j.cgh.2019.04.012
Mack DR, Langton C, Markowitz J, LeLeiko N, Griffiths A, Bousvaros A, Evans J, Kugathasan S, Otley A, Pfefferkorn M, Rosh J, Mezoff A, Moyer S, Oliva-Hemker M, Rothbaum R, Wyllie R, delRosario JF, Keljo D, Lerer T, Hyams J; Pediatric Inflammatory Bowel Disease Collaborative Research Group. Laboratory Values for Children With Newly Diagnosed Inflammatory Bowel Disease. Pediatrics. 2007;119(6):1113-9. https://doi.org/10.1542/peds.2006-1865
Sabery N, Bass D. Use of Serologic Markers as a Screening Tool in Inflammatory Bowel Disease Compared With Elevated Erythrocyte Sedimentation Rate and Anemia. Pediatrics. 2007;119(1):e193-9. https://doi.org/10.1542/peds.2006-1361
Holtman GA, Lisman-van Leeuwen Y, Reitsma JB, Berger MY. Noninvasive Tests for Inflammatory Bowel Disease: A Meta-analysis. Pediatrics. 2016;137(1):e20152126. https://doi.org/10.1542/peds.2015-2126
Prideaux L, De Cruz P, Ng SC, Kamm MA. Serological Antibodies in Inflammatory Bowel Disease: A Systematic Review: Inflamm Bowel Dis. 2012;18(7):1340-55. https://doi.org/10.1002/ibd.21903
Joossens S, Reinisch W, Vermeire S, Sendid B, Poulain D, Peeters M, Geboes K, Bossuyt X, Vandewalle P, Oberhuber G, Vogelsang H, Rutgeerts P, Colombel JF. The value of serologic markers in indeterminate colitis: A prospective follow-up study. Gastroenterology. 2002;122(5):1242-7. https://doi.org/10.1053/gast.2002.32980
Birimberg-Schwartz L, Wilson DC, Kolho KL, Karolewska-Bochenek K, Afzal NA, Spray C, Romano C, Lionetti P, Hauer AC, Martinez-Vinson C, Veres G, Escher JC, Turner D; paediatric IBD Porto group of ESPGHAN. pANCA and ASCA in Children with IBD-Unclassified, Crohn’s Colitis, and Ulcerative Colitis-A Longitudinal Report from the IBD Porto Group of ESPGHAN: Inflamm Bowel Dis. 2016;22(8):1908-14. https://doi.org/10.1097/MIB.0000000000000784
Amre DK, Lu SE, Costea F, Seidman EG. Utility of Serological Markers in Predicting the Early Occurrence of Complications and Surgery in Pediatric Crohn’s Disease Patients. Am J Gastroenterol. 2006;101(3):645-52. https://doi.org/10.1111/j.1572-0241.2006.00468.x
Chang S. Disease monitoring in inflammatory bowel disease. World J Gastroenterol. 2015;21(40):11246. https://doi.org/10.3748/wjg.v21.i40.11246
Koninckx CR, Donat E, Benninga MA, Broekaert IJ, Gottrand F, Kolho KL, Lionetti P, Miele E, Orel R, Papadopoulou A, Pienar C, Schäppi MG, Wilschanski M, Thapar N. The Use of Fecal Calprotectin Testing in Paediatric Disorders: A Position Paper of the European Society for Paediatric Gastroenterology and Nutrition Gastroenterology Committee. J Pediatr Gastroenterol Nutr. 2021;72(4):617-40. https://doi.org/10.1097/MPG.0000000000003046
Kapel N, Campeotto F, Kalach N, Baldassare M, Butel MJ, Dupont C. Faecal Calprotectin in Term and Preterm Neonates. J Pediatr Gastroenterol Nutr. 2010;51(5):542-7. https://doi.org/10.1097/MPG.0b013e3181e2ad72
Turner D, Ricciuto A, Lewis A, D’Amico F, Dhaliwal J, Griffiths AM, Bettenworth D, Sandborn WJ, Sands BE, Reinisch W, Schölmerich J, Bemelman W, Danese S, Mary JY, Rubin D, Colombel JF, Peyrin-Biroulet L, Dotan I, Abreu MT, Dignass A; International Organization for the Study of IBD. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160(5):1570-83. https://doi.org/10.1053/j.gastro.2020.12.031
Miele E, Shamir R, Aloi M, Assa A, Braegger C, Bronsky J, de Ridder L, Escher JC, Hojsak I, Kolaček S, Koletzko S, Levine A, Lionetti P, Martinelli M, Ruemmele F, Russell RK, Boneh RS, van Limbergen J, Veereman G, Staiano A. Nutrition in Pediatric Inflammatory Bowel Disease: A Position Paper on Behalf of the Porto Inflammatory Bowel Disease Group of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66(4):687-708. https://doi.org/10.1097/MPG.0000000000001896
Narula N, Dhillon A, Zhang D, Sherlock ME, Tondeur M, Zachos M. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane IBD Group, editor. Cochrane Database Syst Rev. 2018;4:CD000542. https://doi.org/10.1002/14651858.CD000542.pub3
Rubio A, Pigneur B, Garnier-Lengliné H, Talbotec C, Schmitz J, Canioni D, Goulet O, Ruemmele FM. The efficacy of exclusive nutritional therapy in paediatric Crohn’s disease, comparing fractionated oral vs. continuous enteral feeding: Oral vs. continuous enteral nutrition for CD in children. Aliment Pharmacol Ther. 2011;33(12):1332-9. https://doi.org/10.1111/j.1365-2036.2011.04662.x
Levine A, Wine E, Assa A, Sigall Boneh R, Shaoul R, Kori M, Cohen S, Peleg S, Shamaly H, On A, Millman P, Abramas L, Ziv-Baran T, Grant S, Abitbol G, Dunn KA, Bielawski JP, Van Limbergen J. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology. 2019;157(2):440-450.e8. https://doi.org/10.1053/j.gastro.2019.04.021
Faiman A, Mutalib M, Moylan A, Morgan N, Crespi D, Furman M, Kader A. Standard versus rapid food reintroduction after exclusive enteral nutritional therapy in paediatric Crohn’s disease: Eur J Gastroenterol Hepatol. 2014;26(3):276-81. https://doi.org/10.1097/MEG.0000000000000027
Wong S, Lemberg DA, Day AS. Exclusive enteral nutrition in the management of perianal Crohn’s disease in children: Enteral nutrition in perianal Crohn’s disease. J Dig Dis. 2010;11(3):185-8. https://doi.org/10.1111/j.1751-2980.2010.00434.x
Day AS, Whitten KE, Sidler M, Lemberg DA. Systematic review: nutritional therapy in paediatric Crohn’s disease: SYSTEMATIC REVIEW: NUTRITION IN CHILDREN WITH CROHN’S. Aliment Pharmacol Ther. 2007;27(4):293-307. https://doi.org/10.1111/j.1365-2036.2007.03578.x
Akobeng AK, Thomas AG. Refeeding Syndrome Following Exclusive Enteral Nutritional Treatment in Crohn Disease. J Pediatr Gastroenterol Nutr. 2010;51(3):364-6. https://doi.org/10.1097/MPG.0b013e3181e712d6
Pigneur B, Lepage P, Mondot S, Schmitz J, Goulet O, Doré J, Ruemmele FM. Mucosal Healing and Bacterial Composition in Response to Enteral Nutrition Vs Steroid-based Induction Therapy-A Randomised Prospective Clinical Trial in Children With Crohn’s Disease. J Crohns Colitis. 2019;13(7):846-55. https://doi.org/10.1093/ecco-jcc/jjy207
Grover Z, Muir R, Lewindon P. Exclusive enteral nutrition induces early clinical, mucosal and transmural remission in paediatric Crohn’s disease. J Gastroenterol. 2014;49(4):638-45. https://doi.org/10.1007/s00535-013-0815-0
Urlep D, Benedik E, Brecelj J, Orel R. Partial enteral nutrition induces clinical and endoscopic remission in active pediatric Crohn’s disease: results of a prospective cohort study. Eur J Pediatr. 2020;179(3):431-8. https://doi.org/10.1007/s00431-019-03520-7
Sigall Boneh R, Van Limbergen J, Wine E, Assa A, Shaoul R, Milman P, Cohen S, Kori M, Peleg S, On A, Shamaly H, Abramas L, Levine A. Dietary Therapies Induce Rapid Response and Remission in Pediatric Patients With Active Crohn’s Disease. Clin Gastroenterol Hepatol. 2021;19(4):752-9. https://doi.org/10.1016/j.cgh.2020.04.006
Greenberg GR, Fleming CR, Jeejeebhoy KN, Rosenberg IH, Sales D, Tremaine WJ. Controlled trial of bowel rest and nutritional support in the management of Crohn’s disease. Gut. 1988;29(10):1309-15. https://doi.org/10.1136/gut.29.10.1309
Jones VA. Comparison of total parenteral nutrition and elemental diet in induction of remission of Crohn’s disease: Long-term maintenance of remission by personalized food exclusion diets. Dig Dis Sci. 1987;32(S12):S100-7. https://doi.org/10.1007/BF01312473
Yu Y, Chen KC, Chen J. Exclusive enteral nutrition versus corticosteroids for treatment of pediatric Crohn’s disease: a meta-analysis. World J Pediatr. 2019;15(1):26-36. https://doi.org/10.1007/s12519-018-0204-0
Swaminath A, Feathers A, Ananthakrishnan AN, Falzon L, Li Ferry S. Systematic review with meta-analysis: enteral nutrition therapy for the induction of remission in paediatric Crohn’s disease. Aliment Pharmacol Ther. 2017;46(7):645-56. https://doi.org/10.1111/apt.14253
Levine A, Turner D, Pfeffer Gik T, Amil Dias J, Veres G, Shaoul R, Staiano A, Escher J, Kolho KL, Paerregaard A, Martin de Carpi J, Veereman Wauters G, Koletzko S, Shevah O, Finnby L, Sladek M. Comparison of Outcomes Parameters for Induction of Remission in New Onset Pediatric Crohn’s Disease: Evaluation of the Porto IBD Group “Growth Relapse and Outcomes with Therapy” (GROWTH CD) Study. Inflamm Bowel Dis. 2014;20(2):278-85. https://doi.org/10.1097/01.MIB.0000437735.11953.68
Hart L, Farbod Y, Szamosi JC, Yamamoto M, Britz-McKibbin P, Halgren C, Zachos M, Pai N. Effect of Exclusive Enteral Nutrition and Corticosteroid Induction Therapy on the Gut Microbiota of Pediatric Patients with Inflammatory Bowel Disease. Nutrients. 2020;12(6):1691. https://doi.org/10.3390/nu12061691
Connors J, Basseri S, Grant A, Giffin N, Mahdi G, Noble A, Rashid M, Otley A, Van Limbergen J. Exclusive Enteral Nutrition Therapy in Paediatric Crohn’s Disease Results in Long-term Avoidance of Corticosteroids: Results of a Propensity-score Matched Cohort Analysis. J Crohns Colitis. 2017;11(9):1063-70. https://doi.org/10.1093/ecco-jcc/jjx060
Cohen-Dolev N, Sladek M, Hussey S, Turner D, Veres G, Koletzko S, et al. Differences in Outcomes Over Time With Exclusive Enteral Nutrition Compared With Steroids in Children With Mild to Moderate Crohn’s Disease: Results From the GROWTH CD Study. J Crohns Colitis. 2018;12(3):306-12. https://doi.org/10.1093/ecco-jcc/jjx150
Assa A, Shamir R. Exclusive enteral nutrition for inducing remission in inflammatory bowel disease in paediatric patients. Curr Opin Clin Nutr Metab Care. 2017;20(5):384-9. https://doi.org/10.1097/MCO.0000000000000402
Escher JC. Budesonide versus prednisolone for the treatment of active Crohn’s disease in children: a randomized, double-blind, controlled, multicentre trial. Eur J Gastroenterol Hepatol. 2004;16(1):47-54. https://doi.org/10.1097/00042737-200401000-00008
Sidoroff M, Kolho KL. Glucocorticoids in pediatric inflammatory bowel disease. Scand J Gastroenterol. 2012;47(7):745-50. https://doi.org/10.3109/00365521.2012.679681
Huscher D, Thiele K, Gromnica-Ihle E, Hein G, Demary W, Dreher R, Zink A, Buttgereit F. Dose-related patterns of glucocorticoid-induced side effects. Ann Rheum Dis. 2009;68(7):1119-24. https://doi.org/10.1136/ard.2008.092163
Agrawal A, Durrani S, Leiper K, Ellis A, Morris AI, Rhodes JM. Effect of Systemic Corticosteroid Therapy on Risk for Intra-Abdominal or Pelvic Abscess in Non-operated Crohn’s Disease. Clin Gastroenterol Hepatol. 2005;3(12):1215-20. https://doi.org/10.1016/S1542-3565(05)00759-7
Singh S, Facciorusso A, Dulai PS, Jairath V, Sandborn WJ. Comparative Risk of Serious Infections With Biologic and/or Immunosuppressive Therapy in Patients With Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol. 2020;18(1):69-81.e3. https://doi.org/10.1016/j.cgh.2019.02.044
Costello R, Patel R, Humphreys J, McBeth J, Dixon WG. Patient perceptions of glucocorticoid side effects: a cross-sectional survey of users in an online health community. BMJ Open. 2017;7(4):e014603. https://doi.org/10.1136/bmjopen-2016-014603
Levine A, Kori M, Dinari G, Broide E, Shaoul R, Yerushalmi B, On A, Bujanover Y, Pröls M, Greinwald R; Israeli Pediatric Budesonide Study Group. Comparison of two dosing methods for induction of response and remission with oral budesonide in active pediatric Crohn’s disease: A randomized placebo-controlled trial: Inflamm Bowel Dis. 2009;15(7):1055-61. https://doi.org/10.1002/ibd.20881
Kuenzig ME, Rezaie A, Kaplan GG, Otley AR, Steinhart AH, Griffiths AM, Benchimol EI, Seow CH. Budesonide for the Induction and Maintenance of Remission in Crohn’s Disease: Systematic Review and Meta-Analysis for the Cochrane Collaboration. J Can Assoc Gastroenterol. 2018;1(4):159-73. https://doi.org/10.1093/jcag/gwy018
Levine A, Broide E, Stein M, Bujanover Y, Weizman Z, Dinari G, Pacht A, Branski D, Zahavi I. Evaluation of oral budesonide for treatment of mild and moderate exacerbations of Crohn’s disease in children. J Pediatr. 2002;140(1):75-80. https://doi.org/10.1067/mpd.2002.119992
Hicks CW, Wick EC, Salvatori R, Ha CY. Perioperative Corticosteroid Management for Patients with Inflammatory Bowel Disease: Inflamm Bowel Dis. 2015;21(1):221-8. https://doi.org/10.1097/MIB.0000000000000185
Sidoroff M, Kolho KL. Screening for adrenal suppression in children with inflammatory bowel disease discontinuing glucocorticoid therapy. BMC Gastroenterol. 2014;14(1):51. https://doi.org/10.1186/1471-230X-14-51
Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, Danese S, D’Hoore A, Gassull M, Gomollón F, Hommes DW, Michetti P, O’Morain C, Oresland T, Windsor A, Stange EF, Travis SP; European Crohn’s and Colitis Organisation (ECCO). The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis. 2010;4(1):28-62. https://doi.org/10.1016/j.crohns.2009.12.002
Levine A, Kori M, Kierkus J, Sigall Boneh R, Sladek M, Escher JC, Wine E, Yerushalmi B, Amil Dias J, Shaoul R, Veereman Wauters G, Boaz M, Abitbol G, Bousvaros A, Turner D. Azithromycin and metronidazole versus metronidazole-based therapy for the induction of remission in mild to moderate paediatric Crohn’s disease: a randomised controlled trial. Gut. 2019;68(2):239-47. https://doi.org/10.1136/gutjnl-2017-315199
Townsend CM, Parker CE, MacDonald JK, Nguyen TM, Jairath V, Feagan BG, Khanna R. Antibiotics for induction and maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2019;2(2):CD012730. https://doi.org/10.1002/14651858.CD012730.pub2
Lim WC, Hanauer S. Aminosalicylates for induction of remission or response in Crohn’s disease. En: The Cochrane Collaboration, editor. Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2010. p. CD008870. https://doi.org/10.1002/14651858.CD008870
Sokollik C, Fournier N, Rizzuti D, Braegger CP, Nydegger A, Schibli S, Spalinger J; Swiss IBD Cohort Study Group. The Use of 5-Aminosalicylic Acid in Children and Adolescents With Inflammatory Bowel Disease. J Clin Gastroenterol. 2018;52(10):e87-91. https://doi.org/10.1097/MCG.0000000000000864
Cezard JP, Munck A, Mouterde O, Morali A, Lenaerts C, Lachaux A, Turck D, Schmitz J, Maurage C, Girardet JP, Belli D, Lamireau T, Sarles J, Chouraqui JP, Descos B, Dabadi A, Meyer M, Olives JP, Mary JY. Prevention of relapse by mesalazine (Pentasa®) in pediatric Crohn’s disease: A multicenter, double-blind, randomized, placebo-controlled trial. Gastroentérologie Clin Biol. 2009;33(1):31-40. https://doi.org/10.1016/j.gcb.2008.07.007
Sood A, Ahuja V, Midha V, Sinha SK, Pai CG, Kedia S, Mehta V, Bopanna S, Abraham P, Banerjee R, Bhatia S, Chakravartty K, Dadhich S, Desai D, Dwivedi M, Goswami B, Kaur K, Khosla R, Kumar A, Mahajan R, Misra SP, Peddi K, Singh SP, Singh A. Colitis and Crohn’s Foundation (India) consensus statements on use of 5-aminosalicylic acid in inflammatory bowel disease. Intest Res. 2020;18(4):355-78. https://doi.org/10.5217/ir.2019.09176
Akobeng AK, Zhang D, Gordon M, MacDonald JK. Oral 5-aminosalicylic acid for maintenance of medically-induced remission in Crohn’s disease. Cochrane IBD Group, editor. Cochrane Database Syst Rev. 2016;9(9):CD003715. https://doi.org/10.1002/14651858.CD003715.pub3
Griffiths A, Koletzko S, Sylvester F, Marcon M, Sherman P. Slow-Release 5-Aminosalicylic Acid Therapy in Children with Small Intestinal Crohn’s Disease: J Pediatr Gastroenterol Nutr. 1993;17(2):186-92. https://doi.org/10.1097/00005176-199308000-00010
Sedano Muñoz R, Quera Pino R, Ibáñez Lazo P, Figueroa Corona C, Flores Pérez L. Aminosalicilatos, tiopurínicos y metotrexato en la enfermedad inflamatoria intestinal, ¿es posible suspender el tratamiento? Gastroenterol Hepatol. 2019;42(5):339-47. https://doi.org/10.1016/j.gastrohep.2019.01.013
Markowitz J, Grancher K, Kohn N, Lesser M, Daum F. A multicenter trial of 6-mercaptopurine and prednisone in children with newly diagnosed Crohn’s disease. Gastroenterology. 2000;119(4):895-902. https://doi.org/10.1053/gast.2000.18144
Ashworth LA, Billett A, Mitchell P, Nuti F, Siegel C, Bousvaros A. Lymphoma risk in children and young adults with inflammatory bowel disease: Analysis of a large single-center cohort: Inflamm Bowel Dis. 2012;18(5):838-43. https://doi.org/10.1002/ibd.21844
Shah ED, Coburn ES, Nayyar A, Lee KJ, Koliani-Pace JL, Siegel CA. Systematic review: hepatosplenic T-cell lymphoma on biologic therapy for inflammatory bowel disease, including data from the Food and Drug Administration Adverse Event Reporting System. Aliment Pharmacol Ther. 2020;51(5):527-33. https://doi.org/10.1111/apt.15637
Kotlyar DS, Lewis JD, Beaugerie L, Tierney A, Brensinger CM, Gisbert JP, Loftus EV Jr, Peyrin-Biroulet L, Blonski WC, Van Domselaar M, Chaparro M, Sandilya S, Bewtra M, Beigel F, Biancone L, Lichtenstein GR. Risk of Lymphoma in Patients With Inflammatory Bowel Disease Treated With Azathioprine and 6-Mercaptopurine: A Meta-analysis. Clin Gastroenterol Hepatol. 2015;13(5):847-858.e4. https://doi.org/10.1016/j.cgh.2014.05.015
Mack DR, Benchimol EI, Critch J, deBruyn J, Tse F, Moayyedi P, Church P, Deslandres C, El-Matary W, Huynh H, Jantchou P, Lawrence S, Otley A, Sherlock M, Walters T, Kappelman MD, Sadowski D, Marshall JK, Griffiths A. Canadian Association of Gastroenterology Clinical Practice Guideline for the Medical Management of Pediatric Luminal Crohn’s Disease. Gastroenterology. 2019;157(2):320-48. https://doi.org/10.1053/j.gastro.2019.03.022
Patel V, Wang Y, MacDonald JK, McDonald JW, Chande N. Methotrexate for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2014;2014(8):CD006884. https://doi.org/10.1002/14651858.CD006884.pub3
Colman RJ, Lawton RC, Dubinsky MC, Rubin DT. Methotrexate for the Treatment of Pediatric Crohn’s Disease: A Systematic Review and Meta-analysis. Inflamm Bowel Dis. 2018;24(10):2135-41. https://doi.org/10.1093/ibd/izy078
Ejima-Yamada K, Oshiro Y, Okamura S, Fujisaki T, Mihashi Y, Tamura K, Fukushige T, Kojima M, Shibuya K, Takeshita M. Epstein-Barr virus infection and gene promoter hypermethylation in rheumatoid arthritis patients with methotrexate-associated B cell lymphoproliferative disorders. Virchows Arch. 2017;470(2):205-15. https://doi.org/10.1007/s00428-016-2030-x
Yamamoto T, Nakahigashi M, Umegae S, Matsumoto K. Enteral nutrition for the maintenance of remission in Crohn’s disease: a systematic review: Eur J Gastroenterol Hepatol. 2010;22(1):1-8. https://doi.org/10.1097/MEG.0b013e32832c788c
Takagi S, Utsunomiya K, Kuriyama S, Yokoyama H, Takahashi S, Iwabuchi M, Takahashi H, Takahashi S, Kinouchi Y, Hiwatashi N, Funayama Y, Sasaki I, Tsuji I, Shimosegawa T. Effectiveness of an «half elemental diet» as maintenance therapy for Crohn’s disease: a randomized-controlled trial. Aliment Pharmacol Ther. 2006;24(9):1333-40. https://doi.org/10.1111/j.1365-2036.2006.03120.x
Yamamoto T, Shiraki M, Nakahigashi M, Umegae S, Matsumoto K. Enteral nutrition to suppress postoperative Crohn’s disease recurrence: a five-year prospective cohort study. Int J Colorectal Dis. 2013;28(3):335-40. https://doi.org/10.1007/s00384-012-1587-3
Gkikas K, Gerasimidis K, Milling S, Ijaz UZ, Hansen R, Russell RK. Dietary Strategies for Maintenance of Clinical Remission in Inflammatory Bowel Diseases: Are We There Yet? Nutrients. 2020;12(7):2018. https://doi.org/10.3390/nu12072018
El-Matary W, Otley A, Critch J, Abou-Setta AM. Enteral Feeding Therapy for Maintaining Remission in Crohn’s Disease: A Systematic Review. J Parenter Enter Nutr. 2017;41(4):550-61. https://doi.org/10.1177/0148607115621051
Tsertsvadze A, Gurung T, Court R, Clarke A, Sutcliffe P. Clinical effectiveness and cost-effectiveness of elemental nutrition for the maintenance of remission in Crohn’s disease: a systematic review and meta-analysis. Health Technol Assess. 2015;19(26):1-138. https://doi.org/10.3310/hta19260
Barclay AR, Russell RK, Wilson ML, Gilmour WH, Satsangi J, Wilson DC. Systematic Review: The Role of Breastfeeding in the Development of Pediatric Inflammatory Bowel Disease. J Pediatr. 2009;155(3):421-6. https://doi.org/10.1016/j.jpeds.2009.03.017
Charlebois A, Rosenfeld G, Bressler B. The Impact of Dietary Interventions on the Symptoms of Inflammatory Bowel Disease: A Systematic Review. Crit Rev Food Sci Nutr. 2016;56(8):1370-8. https://doi.org/10.1080/10408398.2012.760515
Penagini F, Dilillo D, Borsani B, Cococcioni L, Galli E, Bedogni G, Zuin G, Zuccotti GV. Nutrition in Pediatric Inflammatory Bowel Disease: From Etiology to Treatment. A Systematic Review. Nutrients. 2016;8(6):334. https://doi.org/10.3390/nu8060334
Racine A, Carbonnel F, Chan SS, Hart AR, Bueno-de-Mesquita HB, Oldenburg B, van Schaik FD, Tjønneland A, Olsen A, Dahm CC, Key T, Luben R, Khaw KT, Riboli E, Grip O, Lindgren S, Hallmans G, Karling P, Clavel-Chapelon F, Bergman MM, Boeing H, Kaaks R, Katzke VA, Palli D, Masala G, Jantchou P, Boutron-Ruault MC. Dietary Patterns and Risk of Inflammatory Bowel Disease in Europe: Results from the EPIC Study. Inflamm Bowel Dis. 2016;22(2):345-54. https://doi.org/10.1097/MIB.0000000000000638
Goyal A, Zheng Y, Albenberg LG, Stoner NL, Hart L, Alkhouri R, Hampson K, Ali S, Cho-Dorado M, Goyal RK, Grossman A. Anemia in Children With Inflammatory Bowel Disease: A Position Paper by the IBD Committee of the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2020;71(4):563-82. https://doi.org/10.1097/MPG.0000000000002885
Tulewicz-Marti E, Moniuszko A, Rydzewska G. Management of anemia in inflammatory bowel disease: a challenge in everyday clinical practice. Gastroenterol Rev. 2017;4:239-43. https://doi.org/10.5114/pg.2017.72096
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for calcium. EFSA J. 2015;13(5):4101. https://doi.org/10.2903/j.efsa.2015.4101
Pappa HM, Mitchell PD, Jiang H, Kassiff S, Filip-Dhima R, DiFabio D, Quinn N, Lawton RC, Varvaris M, Van Straaten S, Gordon CM. Treatment of Vitamin D Insufficiency in Children and Adolescents with Inflammatory Bowel Disease: A Randomized Clinical Trial Comparing Three Regimens. J Clin Endocrinol Metab. 2012;97(6):2134-42. https://doi.org/10.1210/jc.2011-3182
Bousvaros A, Guandalini S, Baldassano RN, Botelho C, Evans J, Ferry GD, Goldin B, Hartigan L, Kugathasan S, Levy J, Murray KF, Oliva-Hemker M, Rosh JR, Tolia V, Zholudev A, Vanderhoof JA, Hibberd PL. A Randomized, Double-blind Trial of Lactobacillus GG Versus Placebo in Addition to Standard Maintenance Therapy for Children with Crohn’s Disease: Inflamm Bowel Dis. 2005;11(9):833-9. https://doi.org/10.1097/01.MIB.0000175905.00212.2c
Rolfe VE, Fortun PJ, Hawkey CJ, Bath-Hextall FJ. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826. https://doi.org/10.1002/14651858.CD004826.pub2
Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;2008(3):CD006634. https://doi.org/10.1002/14651858.CD006634.pub2
Imdad A, Nicholson MR, Tanner-Smith EE, Zackular JP, Gomez-Duarte OG, Beaulieu DB, Acra S. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. 2018;11(11):CD012774. https://doi.org/10.1002/14651858.CD012774.pub2
Fang H, Fu L, Wang J. Protocol for Fecal Microbiota Transplantation in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. BioMed Res Int. 2018;2018:1-11. https://doi.org/10.1155/2018/8941340
Yang C, Singh P, Singh H, Le ML, El-Matary W. Systematic review: thalidomide and thalidomide analogues for treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41(11):1079-93. https://doi.org/10.1111/apt.13181
Lazzerini M, Villanacci V, Pellegrin MC, Martelossi S, Magazzù G, Pellegrino S, Lucanto MC, Barabino A, Calvi A, Arrigo S, Lionetti P, Fontana M, Zuin G, Maggiore G, Bramuzzo M, Maschio M, Salemme M, Manenti S, Lorenzi L, Decorti G, Montico M, Ventura A. Endoscopic and Histologic Healing in Children With Inflammatory Bowel Diseases Treated With Thalidomide. Clin Gastroenterol Hepatol. 2017;15(9):1382-1389.e1. https://doi.org/10.1016/j.cgh.2017.02.029
Kennedy NA, Heap GA, Green HD, Hamilton B, Bewshea C, Walker GJ, Thomas A, Nice R, Perry MH, Bouri S, Chanchlani N, Heerasing NM, Hendy P, Lin S, Gaya DR, Cummings JRF, Selinger CP, Lees CW, Hart AL, Parkes M, Sebastian S, Mansfield JC, Irving PM, Lindsay J, Russell RK, McDonald TJ, McGovern D, Goodhand JR, Ahmad T; UK Inflammatory Bowel Disease Pharmacogenetics Study Group. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4(5):341-53. https://doi.org/10.1016/S2468-1253(19)30012-3
Merras-Salmio L, Kolho KL. Clinical Use of Infliximab Trough Levels and Antibodies to Infliximab in Pediatric Patients With Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr. 2017;64(2):272-8. https://doi.org/10.1097/MPG.0000000000001258
Clarkston K, Tsai YT, Jackson K, Rosen MJ, Denson LA, Minar P. Development of Infliximab Target Concentrations During Induction in Pediatric Crohn Disease Patients. J Pediatr Gastroenterol Nutr. 2019;69(1):68-74. https://doi.org/10.1097/MPG.0000000000002304
Chavannes M, Martinez-Vinson C, Hart L, Kaniki N, Chao CY, Lawrence S, Jacobson K, Hugot JP, Viala J, Deslandres C, Jantchou P, Seidman EG. Management of Paediatric Patients With Medically Refractory Crohn’s Disease Using Ustekinumab: A Multi-Centred Cohort Study. J Crohns Colitis. 2019;13(5):578-84. https://doi.org/10.1093/ecco-jcc/jjy206
Ledder O, Assa A, Levine A, Escher JC, de Ridder L, Ruemmele F, Shah N, Shaoul R, Wolters VM, Rodrigues A, Uhlig HH, Posovszky C, Kolho KL, Jakobsen C, Cohen S, Shouval DS, de Meij T, Martin-de-Carpi J, Richmond L, Bronsky J, Friedman M, Turner D. Vedolizumab in Paediatric Inflammatory Bowel Disease: A Retrospective Multi-Centre Experience From the Paediatric IBD Porto Group of ESPGHAN. J Crohns Colitis. 2017;11(10):1230-7. https://doi.org/10.1093/ecco-jcc/jjx082
de Ridder L, Assa A, Bronsky J, Romano C, Russell RK, Afzal NA, Hauer AC, Knafelz D, Lionetti P, Strisciuglio C, Veres G, Winter H, Wolters VM, Sladek M, Vulto AG, Dias JA; Paediatric IBD Porto group of ESPGHAN. Use of Biosimilars in Paediatric Inflammatory Bowel Disease: A Position Statement of the ESPGHAN Paediatric IBD Porto Group. J Pediatr Gastroenterol Nutr. 2015;61(4):503-8. https://doi.org/10.1097/MPG.0000000000000903
Sassine S, Djani L, Cambron-Asselin C, Savoie M, Lin YF, Qaddouri M, Zekhnine S, Grzywacz K, Groleau V, Dirks M, Drouin É, Halac U, Marchand V, Girard C, Courbette O, Patey N, Dal Soglio D, Deslandres C, Jantchou P. Risk Factors of Clinical Relapses in Pediatric Luminal Crohn’s Disease: A Retrospective Cohort Study. Am J Gastroenterol. 2022;117(4):637-46. https://doi.org/10.14309/ajg.0000000000001650
Boyapati RK, Torres J, Palmela C, Parker CE, Silverberg OM, Upadhyaya SD, Nguyen TM, Colombel JF. Withdrawal of immunosuppressant or biologic therapy for patients with quiescent Crohn’s disease. Cochrane Database Syst Rev. 2018;5(5):CD012540. https://doi.org/10.1002/14651858.CD012540.pub2
Zhang B, Gulati A, Alipour O, Shao L. Relapse From Deep Remission After Therapeutic De-escalation in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J Crohns Colitis. 2020;14(10):1413-23. https://doi.org/10.1093/ecco-jcc/jjaa087
Bemelman WA, Warusavitarne J, Sampietro GM, Serclova Z, Zmora O, Luglio G, de Buck van Overstraeten A, Burke JP, Buskens CJ, Colombo F, Dias JA, Eliakim R, Elosua T, Gecim IE, Kolacek S, Kierkus J, Kolho KL, Lefevre JH, Millan M, Panis Y, Pinkney T, Russell RK, Shwaartz C, Vaizey C, Yassin N, D’Hoore A. ECCO-ESCP Consensus on Surgery for Crohn’s Disease. J Crohns Colitis. 2018;12(1):1-16. https://doi.org/10.1093/ecco-jcc/jjx061
Regueiro M, Feagan BG, Zou B, Johanns J, Blank MA, Chevrier M, Plevy S, Popp J, Cornillie FJ, Lukas M, Danese S, Gionchetti P, Hanauer SB, Reinisch W, Sandborn WJ, Sorrentino D, Rutgeerts P; PREVENT Study Group. Infliximab Reduces Endoscopic, but Not Clinical, Recurrence of Crohn’s Disease After Ileocolonic Resection. Gastroenterology. 2016;150(7):1568-78. https://doi.org/10.1053/j.gastro.2016.02.072
Turner D, Muise AM. Very Early Onset IBD: How Very Different ‘on Average’? J Crohns Colitis. 2017;11(5):517-18. https://doi.org/10.1093/ecco-jcc/jjw217
Kim ES, Kwon Y, Choe YH, Kim MJ. Upper gastrointestinal tract involvement is more prevalent in Korean patients with pediatric Crohn’s disease than in European patients. Sci Rep. diciembre de 2020;10(1):19032. https://doi.org/10.1038/s41598-020-75938-1
Day AS, Lemberg DA. Identification and diagnosis of Crohn disease and ulcerative colitis in children. J Paediatr Child Health. 2020;56(11):1731-4. https://doi.org/10.1111/jpc.14925
Ho SSC, Ross M, Keenan JI, Day AS. Fecal Calprotectin in Combination With Standard Blood Tests in the Diagnosis of Inflammatory Bowel Disease in Children. Front Pediatr. 2021;8:609279. https://doi.org/10.3389/fped.2020.609279
Thomson M, Tringali A, Dumonceau JM, Tavares M, Tabbers MM, Furlano R, Spaander M, Hassan C, Tzvinikos C, Ijsselstijn H, Viala J, Dall’Oglio L, Benninga M, Orel R, Vandenplas Y, Keil R, Romano C, Brownstone E, Hlava Š, Gerner P, Dolak W, Landi R, Huber WD, Everett S, Vecsei A, Aabakken L, Amil-Dias J, Zambelli A. Paediatric Gastrointestinal Endoscopy: European Society for Paediatric Gastroenterology Hepatology and Nutrition and European Society of Gastrointestinal Endoscopy Guidelines. J Pediatr Gastroenterol Nutr. 2017;64(1):133-53. https://doi.org/10.1097/MPG.0000000000001408
Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, Hindryckx P, Langner C, Limdi JK, Pellino G, Zagórowicz E, Raine T, Harbord M, Rieder F; European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017;11(6):649-70. https://doi.org/10.1093/ecco-jcc/jjx008
Norsa L, Ferrari A, Arrigo S, Bramuzzo M, Deganello Saccomani M, Di Nardo G, Illiceto MT, Miele E, Paci M, Romano C, Romeo E, Daperno M, Oliva S; Endoscopy group of the Italian society of pediatric Gastroenterology Hepatology and Nutrition (SIGENP). Scoring Endoscopy in Pediatric Inflammatory Bowel Disease: A Way to Improve Quality. J Pediatr Gastroenterol Nutr. 2021;73(1):48-53. https://doi.org/10.1097/MPG.0000000000003090
Sturm A, Maaser C, Calabrese E, Annese V, Fiorino G, Kucharzik T, Vavricka SR, Verstockt B, van Rheenen P, Tolan D, Taylor SA, Rimola J, Rieder F, Limdi JK, Laghi A, Krustiņš E, Kotze PG, Kopylov U, Katsanos K, Halligan S, Gordon H, González Lama Y, Ellul P, Eliakim R, Castiglione F, Burisch J, Borralho Nunes P, Bettenworth D, Baumgart DC, Stoker J; European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR]. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis. 2019;13(3):273-84. https://doi.org/10.1093/ecco-jcc/jjy114
Löfberg R, Broström O, Karlén P, Tribukait B, Öst Å. Colonoscopic surveillance in long-standing total ulcerative colitis-A 15-year follow-up study. Gastroenterology. 1990;99(4):1021-31. https://doi.org/10.1016/0016-5085(90)90622-8
Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol. 2019;114(3):384-413. https://doi.org/10.14309/ajg.0000000000000152
Magro F, Doherty G, Peyrin-Biroulet L, Svrcek M, Borralho P, Walsh A, Carneiro F, Rosini F, de Hertogh G, Biedermann L, Pouillon L, Scharl M, Tripathi M, Danese S, Villanacci V, Feakins R. ECCO Position Paper: Harmonization of the Approach to Ulcerative Colitis Histopathology. J Crohns Colitis. 2020;14(11):1503-11. https://doi.org/10.1093/ecco-jcc/jjaa110
Clarke WT, Feuerstein JD. Colorectal cancer surveillance in inflammatory bowel disease: Practice guidelines and recent developments. World J Gastroenterol. 2019;25(30):4148-57. https://doi.org/10.3748/wjg.v25.i30.4148
Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA Technical Review on the Diagnosis and Management of Colorectal Neoplasia in Inflammatory Bowel Disease. Gastroenterology. 2010;138(2):746-774.e4. https://doi.org/10.1053/j.gastro.2009.12.035
Mohammed Vashist N, Samaan M, Mosli MH, Parker CE, MacDonald JK, Nelson SA, Zou GY, Feagan BG, Khanna R, Jairath V. Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst Rev. 201816;1(1):CD011450. https://doi.org/10.1002/14651858.CD011450.pub2
Xie T, Zhang T, Ding C, Dai X, Li Y, Guo Z, Wei Y, Gong J, Zhu W, Li J. Ulcerative Colitis Endoscopic Index of Severity (UCEIS) versus Mayo Endoscopic Score (MES) in guiding the need for colectomy in patients with acute severe colitis. Gastroenterol Rep. 2018;6(1):38-44. https://doi.org/10.1093/gastro/gox016
Turner D, Otley AR, Mack D, Hyams J, de Bruijne J, Uusoue K, Walters TD, Zachos M, Mamula P, Beaton DE, Steinhart AH, Griffiths AM. Development, Validation, and Evaluation of a Pediatric Ulcerative Colitis Activity Index: A Prospective Multicenter Study. Gastroenterology. agosto de 2007;133(2):423-32. https://doi.org/10.1053/j.gastro.2007.05.029
Lewis JD, Abreu MT. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology. 2017;152(2):398-414.e6. https://doi.org/10.1053/j.gastro.2016.10.019
Ahmed SF, Horrocks IA, Patterson T, Zaidi S, Ling SC, McGrogan P, Weaver LT. Bone Mineral Assessment by Dual Energy X-ray Absorptiometry in Children With Inflammatory Bowel Disease: Evaluation by Age or Bone Area: J Pediatr Gastroenterol Nutr. 2004;38(3):276-80. https://doi.org/10.1097/00005176-200403000-00008
Lu C, Yang J, Yu W, Li D, Xiang Z, Lin Y, Yu C. Association between 25(OH)D Level, Ultraviolet Exposure, Geographical Location, and Inflammatory Bowel Disease Activity: A Systematic Review and Meta-Analysis. PLoS One. 2015;10(7):e0132036. https://doi.org/10.1371/journal.pone.0132036
Heyman MB, Kierkus J, Spénard J, Shbaklo H, Giguere M. Efficacy and safety of mesalamine suppositories for treatment of ulcerative proctitis in children and adolescents: Inflamm Bowel Dis. 2010;16(11):1931-9. https://doi.org/10.1002/ibd.21256
Hojsak I, Pavić AM, Kolaček S. Mesalamine treatment mimicking relapse in a child with ulcerative colitis. World J Pediatr. 2014;10(4):371-3. https://doi.org/10.1007/s12519-014-0485-x
Orchard TR, van der Geest SAP, Travis SPL. Randomised clinical trial: early assessment after 2 weeks of high-dose mesalazine for moderately active ulcerative colitis - new light on a familiar question: Randomised clinical trial: time to symptom resolution of UC with high-dose 5-ASA. Aliment Pharmacol Ther. 2011;33(9):1028-35. https://doi.org/10.1111/j.1365-2036.2011.04620.x
Turner D, Yerushalmi B, Kori M, Broide E, Mozer-Glassberg Y, Shaoul R, Kolho KL, Shteyer E, Shamaly H, Ledder O, Cohen S, Peleg S, On A, Levine A. Once- Versus Twice-daily Mesalazine to Induce Remission in Paediatric Ulcerative Colitis: A Randomised Controlled Trial. J Crohns Colitis. 2017;11(5):527-33. https://doi.org/10.1093/ecco-jcc/jjw180
Iofel E, Chawla A, Daum F, Markowitz J. Mesalamine Intolerance Mimics Symptoms of Active Inflammatory Bowel Disease: J Pediatr Gastroenterol Nutr. 2002;34(1):73-6. https://doi.org/10.1097/00005176-200201000-00017
Tung J, Loftus EV Jr, Freese DK, El-Youssef M, Zinsmeister AR, Melton LJ 3rd, Harmsen WS, Sandborn WJ, Faubion WA Jr. A population-based study of the frequency of corticosteroid resistance and dependence in pediatric patients with Crohn’s disease and ulcerative colitis: Inflamm Bowel Dis. 2006;12(12):1093-100. https://doi.org/10.1097/01.mib.0000235835.32176.85
Cakir M, Ozgenc F, Yusekkaya HA, Ecevit CO, Yagci RV. Steroid response in moderate to severe pediatric ulcerative colitis: a single center’s experience. World J Pediatr. 2011;7(1):50-3. https://doi.org/10.1007/s12519-011-0245-0
Hyams J, Markowitz J, Lerer T, Griffiths A, Mack D, Bousvaros A, Otley A, Evans J, Pfefferkorn M, Rosh J, Rothbaum R, Kugathasan S, Mezoff A, Wyllie R, Tolia V, delRosario JF, Moyer MS, Oliva-Hemker M, Leleiko N; Pediatric Inflammatory Bowel Disease Collaborative Research Group. The Natural History of Corticosteroid Therapy for Ulcerative Colitis in Children. Clin Gastroenterol Hepatol. 2006;4(9):1118-23. https://doi.org/10.1016/j.cgh.2006.04.008
Bossa F, Fiorella S, Caruso N, Accadia L, Napolitano G, Valvano MR, Andriulli A, Annese V. Continuous Infusion Versus Bolus Administration of Steroids in Severe Attacks of Ulcerative Colitis: A Randomized, Double-Blind Trial. Am J Gastroenterol. 2007;102(3):601-8. https://doi.org/10.1111/j.1572-0241.2006.01007.x
Powell-Tuck J, Bown RL, Lennard-Jones JE. A Comparison of Oral Prednisolone Given as Single or Multiple Daily Doses for Active Proctocolitis. Scand J Gastroenterol. octubre de 1978;13(7):833-7.https://doi.org/10.3109/00365527809182199
D’Haens G. Systematic review: second-generation vs. conventional corticosteroids for induction of remission in ulcerative colitis. Aliment Pharmacol Ther. 2016;44(10):1018-29. https://doi.org/10.1111/apt.13803
Maconi G, Camatta D, Cannatelli R, Ferretti F, Carvalhas Gabrielli A, Ardizzone S. Budesonide MMX in the Treatment of Ulcerative Colitis: Current Perspectives on Efficacy and Safety. Ther Clin Risk Manag. 2021;17:285-92. https://doi.org/10.2147/TCRM.S263835
Danese S, Hart A, Dignass A, Fiorino G, Louis E, Bonovas S, D’Haens G, Dotan I, Rogler G, Paridaens K, Peyrin-Biroulet L. A multicentre prospective cohort study assessing the effectiveness of budesonide MMX® (Cortiment® MMX®) for active, mild‐to‐moderate ulcerative colitis. United Eur Gastroenterol J. 2019;7(9):1171-82. https://doi.org/10.1177/2050640619864848
Lichtenstein GR, Travis S, Danese S, D’Haens G, Moro L, Jones R, Huang M, Ballard ED, Bagin R, Hardiman Y, Collazo R, Sandborn WJ. Budesonide MMX for the Induction of Remission of Mild to Moderate Ulcerative Colitis: A Pooled Safety Analysis. J Crohns Colitis. 2015;9(9):738-46. https://doi.org/10.1093/ecco-jcc/jjv101
Jakobsen C, Bartek J Jr, Wewer V, Vind I, Munkholm P, Groen R, Paerregaard A. Differences in phenotype and disease course in adult and paediatric inflammatory bowel disease - a population-based study: Differences between paediatric and adult IBD. Aliment Pharmacol Ther. 2011;34(10):1217-24. https://doi.org/10.1111/j.1365-2036.2011.04857.x
Church PC, Ho S, Sharma A, Tomalty D, Frost K, Muise A, Walters TD, Griffiths AM. Intensified Infliximab Induction is Associated with Improved Response and Decreased Colectomy in Steroid-Refractory Paediatric Ulcerative Colitis. J Crohns Colitis. 2019;13(8):982-9. ttps://doi.org/10.1093/ecco-jcc/jjz019
Hyams J, Damaraju L, Blank M, Johanns J, Guzzo C, Winter HS, Kugathasan S, Cohen S, Markowitz J, Escher JC, Veereman-Wauters G, Crandall W, Baldassano R, Griffiths A; T72 Study Group. Induction and Maintenance Therapy With Infliximab for Children With Moderate to Severe Ulcerative Colitis. Clin Gastroenterol Hepatol. 2012;10(4):391-399.e1. https://doi.org/10.1016/j.cgh.2011.11.026
Vahabnezhad E, Rabizadeh S, Dubinsky MC. A 10-Year, Single Tertiary Care Center Experience on the Durability of Infliximab in Pediatric Inflammatory Bowel Disease: Inflamm Bowel Dis. 2014;20(4):606-13. https://doi.org/10.1097/MIB.0000000000000003
Volonaki E, Mutalib M, Kiparissi F, Shah N, Lindley KJ, Elawad M. Adalimumab as a second-line biological therapy in children with refractory ulcerative colitis: Eur J Gastroenterol Hepatol. 2015;27(12):1425-8. https://doi.org/10.1097/MEG.0000000000000470
Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D’Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P; SONIC Study Group. Infliximab, Azathioprine, or Combination Therapy for Crohn’s Disease. N Engl J Med. 2010;362(15):1383-95. https://doi.org/10.1056/NEJMoa0904492
Schneider AM, Weghuber D, Hetzer B, Entenmann A, Müller T, Zimmermann G, Schütz S, Huber WD, Pichler J. Vedolizumab use after failure of TNF-α antagonists in children and adolescents with inflammatory bowel disease. BMC Gastroenterol. 2018;18(1):140. https://doi.org/10.1186/s12876-018-0868-x
Jossen J, Kiernan BD, Pittman N, Dubinsky MC. Anti-tumor Necrosis Factor-alpha Exposure Impacts Vedolizumab Mucosal Healing Rates in Pediatric Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr. 2020;70(3):304-9. https://doi.org/10.1097/MPG.0000000000002556
Singh N, Rabizadeh S, Jossen J, Pittman N, Check M, Hashemi G, Phan BL, Hyams JS, Dubinsky MC. Multi-Center Experience of Vedolizumab Effectiveness in Pediatric Inflammatory Bowel Disease: Inflamm Bowel Dis. 2016;22(9):2121-6. https://doi.org/10.1097/MIB.0000000000000865
Abreu MT, Rowbotham DS, Danese S, Sandborn WJ, Miao Y, Zhang H, Tikhonov I, Panaccione R, Hisamatsu T, Scherl EJ, Leong RW, Arasaradnam RP, Afif W, Peyrin-Biroulet L, Sands BE, Marano C. Efficacy and Safety of Maintenance Ustekinumab for Ulcerative Colitis Through 3 Years: UNIFI Long-term Extension. J Crohns Colitis. 2022;16(8):1222-234. https://doi.org/10.1093/ecco-jcc/jjac030
Dayan JR, Dolinger M, Benkov K, Dunkin D, Jossen J, Lai J, Phan BL, Pittman N, Dubinsky MC. Real World Experience With Ustekinumab in Children and Young Adults at a Tertiary Care Pediatric Inflammatory Bowel Disease Center. J Pediatr Gastroenterol Nutr. 2019;69(1):61-7. https://doi.org/10.1097/MPG.0000000000002362
Fusillo SJ, Chang V, Stein RE, Maxwell EC, Conrad MA, Albenberg L, Grossman AB, Mamula B, Piccoli D, Baldassano RN, Kelsen JR. 329 - Ustekinumab Responders versus Non-Responders in Refractory Pediatric Inflammatory Bowel Disease. Gastroenterology. 2018;154(6):S-82. https://doi.org/10.1016/S0016-5085(18)30724-8
Singh N, Rosenthal CJ, Melmed GY, Mirocha J, Farrior S, Callejas S, Tripuraneni B, Rabizadeh S, Dubinsky MC. Early Infliximab Trough Levels Are Associated with Persistent Remission in Pediatric Patients with Inflammatory Bowel Disease: Inflamm Bowel Dis. 2014;20(10):1708-13. https://doi.org/10.1097/MIB.0000000000000137
Papamichael K, Cheifetz AS, Melmed GY, Irving PM, Vande Casteele N, Kozuch PL, Raffals LE, Baidoo L, Bressler B, Devlin SM, Jones J, Kaplan GG, Sparrow MP, Velayos FS, Ullman T, Siegel CA. Appropriate Therapeutic Drug Monitoring of Biologic Agents for Patients With Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2019;17(9):1655-1668.e3. https://doi.org/10.1016/j.cgh.2019.03.037
Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology. 2017;153(3):827-34. https://doi.org/10.1053/j.gastro.2017.07.032
Navas-López VM, Blasco Alonso J, Serrano Nieto MJ, Girón Fernández-Crehuet F, Argos Rodriguez MD, Sierra Salinas C. Oral tacrolimus for pediatric steroid-resistant ulcerative colitis. J Crohns Colitis. 2014;8(1):64-9. https://doi.org/10.1016/j.crohns.2013.03.006
Lawrance IC, Baird A, Lightower D, Radford-Smith G, Andrews JM, Connor S. Efficacy of Rectal Tacrolimus for Induction Therapy in Patients With Resistant Ulcerative Proctitis. Clin Gastroenterol Hepatol. 2017;15(8):1248-55. https://doi.org/10.1016/j.cgh.2017.02.027
Ikeda H, Ishimaru Y, Takayasu H, Fujino J, Kisaki Y, Otani Y, Yamagishi J, Tahara K. Efficacy of Granulocyte Apheresis in Pediatric Patients With Ulcerative Colitis: A Pilot Study. J Pediatr Gastroenterol Nutr. 2006;43(5):592-6. https://doi.org/10.1097/01.mpg.0000237928.07729.79
Martín de Carpi J, Vilar P, Prieto G, Novo MDG, Ribes C, Varea V. Safety and Efficacy of Granulocyte and Monocyte Adsorption Apheresis in Paediatric Inflammatory Bowel Disease: A Prospective Pilot Study. J Pediatr Gastroenterol Nutr. 2008;46(4):386-91. https://doi.org/10.1097/MPG.0b013e31815604e5
Tanaka T, Okanobu H, Kuga Y, Yoshifuku Y, Fujino H, Miwata T, Moriya T, Nishida T, Oya T. Clinical and endoscopic features of responders and non-responders to adsorptive leucocytapheresis: A report based on 120 patients with active ulcerative colitis. Gastroentérologie Clin Biol. 2010;34(12):687-95. https://doi.org/10.1016/j.gcb.2010.08.007
Tomomasa T, Tajiri H, Kagimoto S, Shimizu T, Yoden A, Ushijima K, Uchida K, Kaneko H, Abukawa D, Konno M, Maisawa S, Kohsaka T, Kobayashi A; Japanese Study Group for Pediatric Ulcerative Colitis. Leukocytapheresis in Pediatric Patients With Ulcerative Colitis. J Pediatr Gastroenterol Nutr. 2011;53(1):34-9. https://doi.org/10.1097/MPG.0b013e31821058bc
Thanaraj S, Hamlin PJ, Ford AC. Systematic review: granulocyte/monocyte adsorptive apheresis for ulcerative colitis: Aliment Pharmacol Ther. 2010;32(11-12):1297-306. https://doi.org/10.1111/j.1365-2036.2010.04490.x
Henker J, Müller S, Laass M, Schreiner A, Schulze J. Probiotic Escherichia coli Nissle 1917 (EcN) for Successful Remission Maintenance of Ulcerative Colitis in Children and Adolescents: an Open-Label Pilot Study. Z Für Gastroenterol. 2008;46(09):874-5. https://doi.org/10.1055/s-2008-1027463
Kruis W. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53(11):1617-23. https://doi.org/10.1136/gut.2003.037747
Losurdo G, Iannone A, Contaldo A, Ierardi E, Di Leo A, Principi M. Escherichia coli Nissle 1917 in Ulcerative Colitis Treatment: Systematic Review and Meta-analysis. J Gastrointestin Liver Dis. 2015;24(4):499-505. https://doi.org/10.15403/jgld.2014.1121.244.ecn
Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a Probiotic Preparation (VSL#3) on Induction and Maintenance of Remission in Children With Ulcerative Colitis. Am J Gastroenterol. 2009;104(2):437-43. https://doi.org/10.1038/ajg.2008.118
Huynh HQ, deBruyn J, Guan L, Diaz H, Li M, Girgis S, Turner J, Fedorak R, Madsen K. Probiotic preparation VSL#3 induces remission in children with mild to moderate acute ulcerative colitis: A pilot study: Inflamm Bowel Dis. 2009;15(5):760-8. https://doi.org/10.1002/ibd.20816
Kaur L, Gordon M, Baines PA, Iheozor-Ejiofor Z, Sinopoulou V, Akobeng AK. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2020;3(3):CD005573. https://doi.org/10.1002/14651858.CD005573.pub3
Abraham B, Quigley EMM. Antibiotics and probiotics in inflammatory bowel disease: when to use them? Frontline Gastroenterol. 2020;11(1):62-9. https://doi.org/10.1136/flgastro-2018-101057
Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: A double-blind, placebo-controlled trial. Gastroenterology. 2000;119(2):305-9. https://doi.org/10.1053/gast.2000.9370
Mimura T. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53(1):108-14. https://doi.org/10.1136/gut.53.1.108
Pronio A, Montesani C, Butteroni C, Vecchione S, Mumolo G, Vestri A, Vitolo D, Boirivant M. Probiotic administration in patients with ileal pouch-anal anastomosis for ulcerative colitis is associated with expansion of mucosal regulatory cells: Inflamm Bowel Dis. 2008;14(5):662-8. https://doi.org/10.1002/ibd.20369
Landy J, Hart A. Commentary: the effects of probiotics on barrier function and mucosal pouch microbiota during maintenance treatment for severe pouchitis in patients with ulcerative colitis. Aliment Pharmacol Ther. 2013;38(11-12):1405-6. https://doi.org/10.1111/apt.12517
Goulart RA, Barbalho SM, Lima VM, Souza GA, Matias JN, Araújo AC, Rubira CJ, Buchaim RL, Buchaim DV, Carvalho ACA, Guiguer ÉL. Effects of the Use of Curcumin on Ulcerative Colitis and Crohn’s Disease: A Systematic Review. J Med Food. 2021;24(7):675-85. https://doi.org/10.1089/jmf.2020.0129
Suskind DL, Wahbeh G, Burpee T, Cohen M, Christie D, Weber W. Tolerability of Curcumin in Pediatric Inflammatory Bowel Disease: A Forced-Dose Titration Study. J Pediatr Gastroenterol Nutr. 2013;56(3):277-9. https://doi.org/10.1097/MPG.0b013e318276977d
Hanai H, Iida T, Takeuchi K, Watanabe F, Maruyama Y, Andoh A, Tsujikawa T, Fujiyama Y, Mitsuyama K, Sata M, Yamada M, Iwaoka Y, Kanke K, Hiraishi H, Hirayama K, Arai H, Yoshii S, Uchijima M, Nagata T, Koide Y. Curcumin Maintenance Therapy for Ulcerative Colitis: Randomized, Multicenter, Double-Blind, Placebo-Controlled Trial. Clin Gastroenterol Hepatol. 2006;4(12):1502-6. https://doi.org/10.1016/j.cgh.2006.08.008
Lang A, Salomon N, Wu JC, Kopylov U, Lahat A, Har-Noy O, Ching JY, Cheong PK, Avidan B, Gamus D, Kaimakliotis I, Eliakim R, Ng SC, Ben-Horin S. Curcumin in Combination With Mesalamine Induces Remission in Patients With Mild-to-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2015;13(8):1444-1449.e1. https://doi.org/10.1016/j.cgh.2015.02.019
Kunde S, Pham A, Bonczyk S, Crumb T, Duba M, Conrad H Jr, Cloney D, Kugathasan S. Safety, Tolerability, and Clinical Response After Fecal Transplantation in Children and Young Adults With Ulcerative Colitis. J Pediatr Gastroenterol Nutr. 2013;56(6):597-601. https://doi.org/10.1097/MPG.0b013e318292fa0d
Hourigan SK, Oliva-Hemker M. Fecal microbiota transplantation in children: a brief review. Pediatr Res. 2016;80(1):2-6. https://doi.org/10.1038/pr.2016.48
Turner D, Griffiths AM. Acute severe ulcerative colitis in children: A systematic review: Inflamm Bowel Dis. 2011;17(1):440-9. https://doi.org/10.1002/ibd.21383
Turner D, Mack D, Leleiko N, Walters TD, Uusoue K, Leach ST, Day AS, Crandall W, Silverberg MS, Markowitz J, Otley AR, Keljo D, Mamula P, Kugathasan S, Hyams J, Griffiths AM. Severe Pediatric Ulcerative Colitis: A Prospective Multicenter Study of Outcomes and Predictors of Response. Gastroenterology. 2010;138(7):2282-91. https://doi.org/10.1053/j.gastro.2010.02.047
Turner D, Hyams J, Markowitz J, Lerer T, Mack DR, Evans J, Pfefferkorn M, Rosh J, Kay M, Crandall W, Keljo D, Otley AR, Kugathasan S, Carvalho R, Oliva-Hemker M, Langton C, Mamula P, Bousvaros A, LeLeiko N, Griffiths AM; Pediatric IBD Collaborative Research Group. Appraisal of the pediatric ulcerative colitis activity index (PUCAI): Inflamm Bowel Dis. 2009;15(8):1218-23. https://doi.org/10.1002/ibd.20867
Hourigan SK, Oliva-Hemker M, Hutfless S. The Prevalence of Clostridium difficile Infection in Pediatric and Adult Patients with Inflammatory Bowel Disease. Dig Dis Sci. 2014;59(9):2222-7. https://doi.org/10.1007/s10620-014-3169-4
Cohen S, Martinez-Vinson C, Aloi M, Turner D, Assa A, de Ridder L, Wolters VM, de Meij T, Alvisi P, Bronsky J, Kopylov U; Pediatric IBD Porto Group of ESPGHAN. Cytomegalovirus Infection in Pediatric Severe Ulcerative Colitis-A Multicenter Study from the Pediatric Inflammatory Bowel Disease Porto Group of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. Pediatr Infect Dis J. 2018;37(3):197-201. https://doi.org/10.1097/INF.0000000000001724
Turner D, Walsh CM, Benchimol EI, Mann EH, Thomas KE, Chow C, McLernon RA, Walters TD, Swales J, Steinhart AH, Griffiths AM. Severe paediatric ulcerative colitis: incidence, outcomes and optimal timing for second-line therapy. Gut. 2008;57(3):331-8. https://doi.org/10.1136/gut.2007.136481
Schechter A, Griffiths C, Gana JC, Shaoul R, Shamir R, Shteyer E, Bdolah-Abram T, Ledder O, Turner D. Early endoscopic, laboratory and clinical predictors of poor disease course in paediatric ulcerative colitis. Gut. 2015;64(4):580-8. https://doi.org/10.1136/gutjnl-2014-306999
Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, et al. Guidelines for Diagnosis, Treatment, and Prevention of Clostridium difficile Infections. Am J Gastroenterol. abril de 2013;108(4):478-98. https://doi.org/10.1038/ajg.2013.4
Planche TD, Davies KA, Coen PG, Finney JM, Monahan IM, Morris KA, O’Connor L, Oakley SJ, Pope CF, Wren MW, Shetty NP, Crook DW, Wilcox MH. Differences in outcome according to Clostridium difficile testing method: a prospective multicentre diagnostic validation study of C difficile infection. Lancet Infect Dis. 2013;13(11):936-45. https://doi.org/10.1016/S1473-3099(13)70200-7
Zidar N, Ferkolj I, Tepeš K, Štabuc B, Kojc N, Uršič T, Petrovec M. Diagnosing cytomegalovirus in patients with inflammatory bowel disease-by immunohistochemistry or polymerase chain reaction? Virchows Arch. 2015;466(5):533-9. https://doi.org/10.1007/s00428-015-1741-8
Turner D, Bishai J, Reshef L, Abitbol G, Focht G, Marcus D, Ledder O, Lev-Tzion R, Orlanski-Meyer E, Yerushalmi B, Aloi M, Griffiths AM, Albenberg L, Kolho KL, Assa A, Cohen S, Gophna U, Vlamakis H, Lurz E, Levine A. Antibiotic Cocktail for Pediatric Acute Severe Colitis and the Microbiome: The PRASCO Randomized Controlled Trial. Inflamm Bowel Dis. 2020;26(11):1733-42. https://doi.org/10.1093/ibd/izz298
Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y, Cottone M, de Ridder L, Doherty G, Ehehalt R, Esteve M, Katsanos K, Lees CW, Macmahon E, Moreels T, Reinisch W, Tilg H, Tremblay L, Veereman-Wauters G, Viget N, Yazdanpanah Y, Eliakim R, Colombel JF; European Crohn’s and Colitis Organisation (ECCO). Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8(6):443-68. https://doi.org/10.1016/j.crohns.2013.12.013
Moninuola OO, Milligan W, Lochhead P, Khalili H. Systematic review with meta-analysis: association between acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) and risk of Crohn’s disease and ulcerative colitis exacerbation. Aliment Pharmacol Ther. 2018;47(11):1428-39. https://doi.org/10.1111/apt.14606
Long MD, Kappelman MD, Martin CF, Chen W, Anton K, Sandler RS. Role of Nonsteroidal Anti-Inflammatory Drugs in Exacerbations of Inflammatory Bowel Disease. J Clin Gastroenterol. 2016;50(2):152-6. https://doi.org/10.1097/MCG.0000000000000421
Long MD, Barnes EL, Herfarth HH, Drossman DA. Narcotic use for inflammatory bowel disease and risk factors during hospitalization: Inflamm Bowel Dis. 2012;18(5):869-76. https://doi.org/10.1002/ibd.21806
Nguyen GC, Bernstein CN, Bitton A, Chan AK, Griffiths AM, Leontiadis GI, Geerts W, Bressler B, Butzner JD, Carrier M, Chande N, Marshall JK, Williams C, Kearon C. Consensus Statements on the Risk, Prevention, and Treatment of Venous Thromboembolism in Inflammatory Bowel Disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146(3):835-848.e6. https://doi.org/10.1053/j.gastro.2014.01.042
Kappelman MD, Horvath-Puho E, Sandler RS, Rubin DT, Ullman TA, Pedersen L, Baron JA, Sørensen HT. Thromboembolic risk among Danish children and adults with inflammatory bowel diseases: a population-based nationwide study. Gut. 2011;60(7):937-43. https://doi.org/10.1136/gut.2010.228585
Lazzerini M, Bramuzzo M, Maschio M, Martelossi S, Ventura A. Thromboembolism in pediatric inflammatory bowel disease: Systematic review: Inflamm Bowel Dis. 2011;17(10):2174-83. https://doi.org/10.1002/ibd.21563
Choshen S, Finnamore H, Auth MK, Bdolah-Abram T, Shteyer E, Mack D, Hyams J, Leleiko N, Griffiths A, Turner D. Corticosteroid Dosing in Pediatric Acute Severe Ulcerative Colitis: A Propensity Score Analysis. J Pediatr Gastroenterol Nutr. 2016;63(1):58-64. https://doi.org/10.1097/MPG.0000000000001079
Vora R, Finnamore HE, Crook K, Baillie C, Whittle E, Krishnamurthy B, Venkatesh K, Auth MK. Clinical Experience of Use of High-dose Intravenous Methylprednisolone in Children With Acute Moderate to Severe Colitis. J Pediatr Gastroenterol Nutr. 2016;63(1):51-7. https://doi.org/10.1097/MPG.0000000000001080
Livshits A, Fisher D, Hadas I, Bdolah-Abram T, Mack D, Hyams J, Crandall W, Griffiths AM, Turner D. Abdominal X-ray in Pediatric Acute Severe Colitis and Radiographic Predictors of Response to Intravenous Steroids. J Pediatr Gastroenterol Nutr. 2016;62(2):259-63. https://doi.org/10.1097/MPG.0000000000000910
Benchimol EI, Turner D, Mann EH, Thomas KE, Gomes T, McLernon RA, Griffiths AM. Toxic Megacolon in Children With Inflammatory Bowel Disease: Clinical and Radiographic Characteristics. Am J Gastroenterol. 2008;103(6):1524-31. https://doi.org/10.1111/j.1572-0241.2008.01807.x
Ungar B, Mazor Y, Weisshof R, Yanai H, Ron Y, Goren I, Waizbard A, Yavzori M, Fudim E, Picard O, Loebstein R, Kopylov U, Dotan I, Chowers Y, Eliakim R, Ben-Horin S. Induction infliximab levels among patients with acute severe ulcerative colitis compared with patients with moderately severe ulcerative colitis. Aliment Pharmacol Ther. 2016;43(12):1293-9. https://doi.org/10.1111/apt.13631
Rosen MJ, Minar P, Vinks AA. Review article: applying pharmacokinetics to optimise dosing of anti-TNF biologics in acute severe ulcerative colitis. Aliment Pharmacol Ther. 2015;41(11):1094-103. https://doi.org/10.1111/apt.13175
Hyams JS, Lerer T, Griffiths A, Pfefferkorn M, Stephens M, Evans J, Otley A, Carvalho R, Mack D, Bousvaros A, Rosh J, Grossman A, Tomer G, Kay M, Crandall W, Oliva-Hemker M, Keljo D, LeLeiko N, Markowitz J; Pediatric Inflammatory Bowel Disease Collaborative Research Group. Outcome Following Infliximab Therapy in Children With Ulcerative Colitis. Am J Gastroenterol. 2010;105(6):1430-6. https://doi.org/10.1038/ajg.2009.759
Hyams JS, Lerer T, Mack D, Bousvaros A, Griffiths A, Rosh J, Otley A, Evans J, Stephens M, Kay M, Keljo D, Pfefferkorn M, Saeed S, Crandall W, Michail S, Kappelman MD, Grossman A, Samson C, Sudel B, Oliva-Hemker M, Leleiko N, Markowitz J; Pediatric Infl ammatory Bowel Disease Collaborative Research Group Registry. Outcome Following Thiopurine Use in Children With Ulcerative Colitis: A Prospective Multicenter Registry Study. Am J Gastroenterol. 2011;106(5):981-7. https://doi.org/10.1038/ajg.2010.493
Hyams JS, Davis S, Mack DR, Boyle B, Griffiths AM, LeLeiko NS, Sauer CG, Keljo DJ, Markowitz J, Baker SS, Rosh J, Baldassano RN, Patel A, Pfefferkorn M, Otley A, Heyman M, Noe J, Oliva-Hemker M, Rufo P, Strople J, Ziring D, Guthery SL, Sudel B, Benkov K, Wali P, Moulton D, Evans J, Kappelman MD, Marquis A, Sylvester FA, Collins MH, Venkateswaran S, Dubinsky M, Tangpricha V, Spada KL, Britt A, Saul B, Gotman N, Wang J, Serrano J, Kugathasan S, Walters T, Denson LA. Factors associated with early outcomes following standardised therapy in children with ulcerative colitis (PROTECT): a multicentre inception cohort study. Lancet Gastroenterol Hepatol. 2017;2(12):855-68. https://doi.org/10.1016/S2468-1253(17)30252-2
Whaley KG, Rosen MJ. Contemporary Medical Management of Acute Severe Ulcerative Colitis. Inflamm Bowel Dis. 2019;25(1):56-66. https://doi.org/10.1093/ibd/izy208
Esposito S, Antoniol G, Labate M, Passadore L, Alvisi P, Daccò V, Ghizzi C, Colombo C, Principi N. Vaccines in Children with Inflammatory Bowel Disease: Brief Review. Vaccines. 2021;9(5):487. https://doi.org/10.3390/vaccines9050487
Maus MV, Lionakis MS. Infections associated with the new ‘nibs and mabs’ and cellular therapies. Curr Opin Infect Dis. 2020;33(4):281-9. https://doi.org/10.1097/QCO.0000000000000656
Ashton JJ, Mossotto E, Stafford IS, Haggarty R, Coelho TAF, Batra A, Afzal NA, Mort M, Bunyan D, Beattie RM, Ennis S. Genetic Sequencing of Pediatric Patients Identifies Mutations in Monogenic Inflammatory Bowel Disease Genes that Translate to Distinct Clinical Phenotypes. Clin Transl Gastroenterol. 2020;11(2):e00129. https://doi.org/10.14309/ctg.0000000000000129
Nambu R, Warner N, Mulder DJ, Kotlarz D, McGovern DPB, Cho J, Klein C, Snapper SB, Griffiths AM, Iwama I, Muise AM. A Systematic Review of Monogenic Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2022;20(4):e653-e663. https://doi.org/10.1016/j.cgh.2021.03.021
Janssen LMA, van der Flier M, de Vries E. Lessons Learned From the Clinical Presentation of Common Variable Immunodeficiency Disorders: A Systematic Review and Meta-Analysis. Front Immunol. 2021;12:620709. https://doi.org/10.3389/fimmu.2021.620709
Uhlig HH, Schwerd T, Koletzko S, Shah N, Kammermeier J, Elkadri A, Ouahed J, Wilson DC, Travis SP, Turner D, Klein C, Snapper SB, Muise AM; COLORS in IBD Study Group and NEOPICS. The Diagnostic Approach to Monogenic Very Early Onset Inflammatory Bowel Disease. Gastroenterology. 2014;147(5):990-1007.e3. https://doi.org/10.1053/j.gastro.2014.07.023
Tegtmeyer D, Seidl M, Gerner P, Baumann U, Klemann C. Inflammatory bowel disease caused by primary immunodeficiencies-Clinical presentations, review of literature, and proposal of a rational diagnostic algorithm. Pediatr Allergy Immunol. 2017;28(5):412-29. https://doi.org/10.1111/pai.12734
Abraham RS. How to evaluate for immunodeficiency in patients with autoimmune cytopenias: laboratory evaluation for the diagnosis of inborn errors of immunity associated with immune dysregulation. Hematology. 2020;2020(1):661-72. https://doi.org/10.1182/hematology.2020000173
D’Elios MM, Baldari CT, Annunziato F. Cellular Primary Immunodeficiencies Rare Diseases of the Immune System. 1.a edición. Springer; 2021. https://doi.org/10.1007/978-3-030-70107-9_1
Uhlig HH, Charbit-Henrion F, Kotlarz D, Shouval DS, Schwerd T, Strisciuglio C, de Ridder L, van Limbergen J, Macchi M, Snapper SB, Ruemmele FM, Wilson DC, Travis SPL, Griffiths AM, Turner D, Klein C, Muise AM, Russell RK; Paediatric IBD Porto group of ESPGHAN. Clinical Genomics for the Diagnosis of Monogenic Forms of Inflammatory Bowel Disease: A Position Paper From the Paediatric IBD Porto Group of European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2021;72(3):456-73. https://doi.org/10.1097/MPG.0000000000003017
van Rheenen PF, Aloi M, Biron IA, Carlsen K, Cooney R, Cucchiara S, Cullen G, Escher JC, Kierkus J, Lindsay JO, Roma E, Russell RK, Sieczkowska-Golub J, Harbord M. European Crohn’s and Colitis Organisation Topical Review on Transitional Care in Inflammatory Bowel Disease. J Crohns Colitis. 2017;11(9):1032-8. https://doi.org/10.1093/ecco-jcc/jjx010

Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2023 Revista colombiana de Gastroenterología

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
Los autores/as ceden sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los contenidos están protegidos bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.

| Estadísticas de artículo | |
|---|---|
| Vistas de resúmenes | |
| Vistas de PDF | |
| Descargas de PDF | |
| Vistas de HTML | |
| Otras vistas | |